| 81% |
|
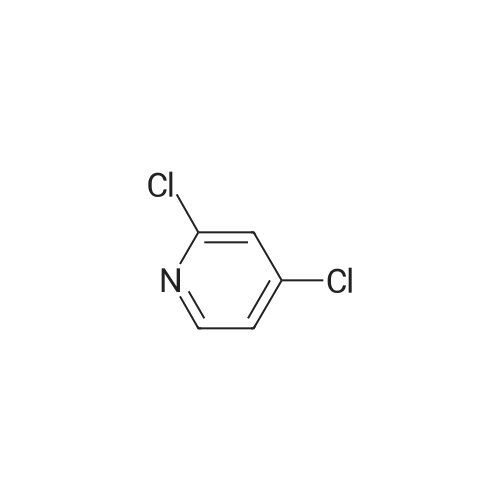
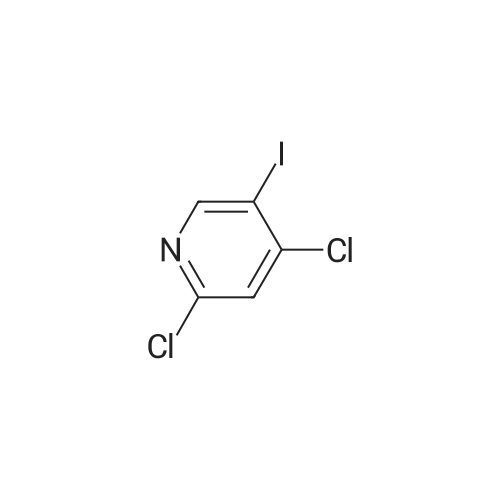
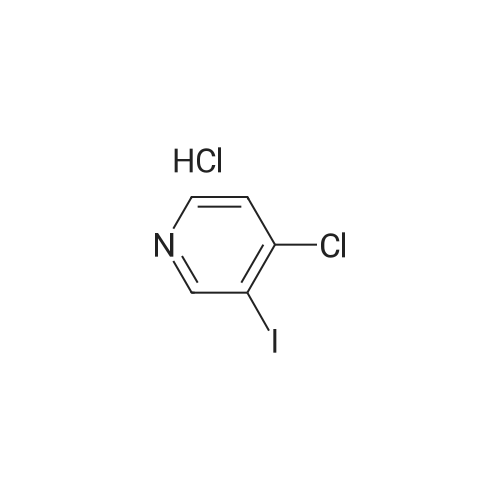
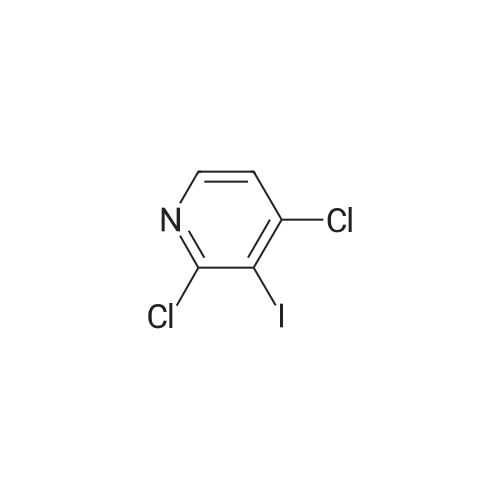
2,4-Dichloro-3 -iodo-pyridine (1-5)To a solution of 2,4-dichloropyridine (5.2 g, 35.14 mmol) and DIPEA (3.91 g, 38.65 mmol) in dry THF (40 mL) cooled at -78 C under a nitrogen atmosphere, was added n-BuLi (24.16 mL, 38.65 mmol, 1.6 M in hexanes) dropwise. The resulting r.m. was stirred at -78 C for 45 min and then a solution of iodine (9.81 g, 38.651 mmol) in dry THF (20 mL) was added dropwise. The mixture was stirred at -78 C for 1 h, allowed to warm to r. , diluted with EtOAc and quenched with H4CI (aq. sat. sol.) and Na2S203 (aq. sat. sol). The organic layer was separated, washed with NaHC03 (aq. sat. sol), dried (Na2S04) and concentrated in vacuo. The crude product was purified by column chromatography (silica gel; Heptane/DCM up to 20% as eluent). The desired fractions were collected and concentrated in vacuo to yield intermediate 1-5 (7.8 g, 81%). |
| 81% |
|
Intermediate 1 (1-1)2,4-Dichloro-3-iodo-pyridine (1-1)To a solution of 2,4-dichloropyridine (5.2 g, 35.14 mmol) and diisopropylamine (3.91 g, 38.65 mmol) in dry THF (40 mL) cooled at -78 C under a nitrogen atmosphere, was added n-butyllithium (24.16 mL, 38.65 mmol, 1.6 M in hexanes) dropwise. The resulting reaction mixture was stirred at -78 C for 45 min. and then a solution of iodine (9.81 g, 38.651 mmol) in dry THF (20 mL) was added dropwise. The mixture was stirred at -78 C for 1 h., allowed to warm to r.t., diluted with EtOAc and quenched with H4CI (aqueous sat. solution) and Na2S203 (aqueous sat. solution). The organic layer was separated, washed with NaHC03 (aqueous sat. solution), dried (Na2S04) and concentrated in vacuo. The crude product was purified by column chromatography (silica gel; DCM in heptane 0/100 to 20/80). The desired fractions were collected and concentrated in vacuo to yield intermediate compound 1-1(7.8 g, 81%). |
| 81% |
|
Intermediate 7 (1-7)2,4-Dichloro-3 -iodo-pyridine (1-7) To a solution of 2,4-dichloropyridine (5.2 g, 35.14 mmol) and DIPEA (3.91 g,38.65 mmol) in dry THF (40 mL) cooled at -78 C under a nitrogen atmosphere, was added n-butyllithium (24.16 mL, 38.65 mmol, 1.6 M in hexanes) dropwise. The resulting reaction mixture was stirred at -78 C for 45 min. and then a solution of iodine (9.81 g, 38.651 mmol) in dry THF (20 mL) was added dropwise. The mixture was stirred at -78 C for 1 h., allowed to warm to r.t., diluted with EtOAc and quenched with H4CI (aqueous sat. solution) and Na2S203 (aqueous sat. solution). The organic layer was separated, washed with NaHC03 (aqueous sat. solution), dried (Na2S04) and concentrated in vacuo. The crude product was purified by column chromatography (silica gel; DCM in heptane 0/100 to 20/80). The desired fractions were collected and concentrated in vacuo to yield intermediate compound 1-7 (7.8 g, 81%). |
| 81% |
|
Intermediate 1 (1-1)2,4-Dichloro-3-iodo-pyridine (1-1)To a solution of 2,4-dichloropyridine (5.2 g, 35.14 mmol) and diisopropylamine (3.91 g, 38.65 mmol) in dry THF (40 mL) cooled at -78 C under a nitrogen atmosphere, was added n-butyllithium (24.16 mL, 38.65 mmol, 1.6 M in hexanes) dropwise. The resulting reaction mixture was stirred at -78 C for 45 min. and then a solution of iodine (9.81 g, 38.651 mmol) in dry THF (20 mL) was added dropwise. The mixture was stirred at -78 C for 1 h., allowed to warm to r.t., diluted with EtOAc and quenched with H4CI (aqueous sat. solution) and Na2S203 (aqueous sat. solution). The organic layer was separated, washed with NaHC03 (aqueous sat. solution), dried (Na2S04) and concentrated in vacuo. The crude product was purified by column chromatography (silica gel; DCM in heptane 0/100 to 20/80). The desired fractions were collected and concentrated in vacuo to yield intermediate compound 1-1 (7.8 g, 81%). |
| 81% |
With n-butyllithium; iodine; diisopropylamine; In tetrahydrofuran; n-heptane; at -78℃; for 1.75h;Inert atmosphere; |
2,4-Dichloro-3-iodo-pyridine (I-1) To a solution of 2,4-dichloropyridine (5.2 g, 35.14 mmol) and diisopropylamine (3.91 g, 38.65 mmol) in dry THF (40 mL) cooled at -78 C. under a nitrogen atmosphere, was added n-butyllithium (24.16 mL, 38.65 mmol, 1.6 M in hexanes) dropwise. The resulting reaction mixture was stirred at -78 C. for 45 min. and then a solution of iodine (9.81 g, 38.651 mmol) in dry THF (20 mL) was added dropwise. The mixture was stirred at -78 C. for 1 h., allowed to warm to r.t., diluted with EtOAc and quenched with NH4Cl (aqueous sat. solution) and Na2S2O3 (aqueous sat. solution). The organic layer was separated, washed with NaHCO3 (aqueous sat. solution), dried (Na2SO4) and concentrated in vacuo. The crude product was purified by column chromatography (silica gel; DCM in heptane 0/100 to 20/80). The desired fractions were collected and concentrated in vacuo to yield intermediate compound I-1 (7.8 g, 81%). |
| 81% |
|
2,4-Dichloro-3-iodo-pyridine (I-5) To a solution of 2,4-dichloropyridine (5.2 g, 35.14 mmol) and DIPEA (3.91 g, 38.65 mmol) in dry THF (40 mL) cooled at -78 C. under a nitrogen atmosphere, was added n-BuLi (24.16 mL, 38.65 mmol, 1.6 M in hexanes) dropwise. The resulting r.m. was stirred at -78 C. for 45 min and then a solution of iodine (9.81 g, 38.651 mmol) in dry THF (20 mL) was added dropwise. The mixture was stirred at -78 C. for 1 h, allowed to warm to r.t., diluted with EtOAc and quenched with NH4Cl (aq. sat. sol.) and Na2S2O3 (aq. sat. sol.). The organic layer was separated, washed with NaHCO3 (aq. sat. sol.), dried (Na2SO4) and concentrated in vacuo. The crude product was purified by column chromatography (silica gel; Heptane/DCM up to 20% as eluent). The desired fractions were collected and concentrated in vacuo to yield intermediate I-5 (7.8 g, 81%). |
| 81% |
|
To a solution of 2,4-dichloropyridine (5.2 g, 35.137 mmol) and diisopropylamine (3.911 g, 38.651 mmol) in dry THF (40 ml) cooled at -78 C under a nitrogen atmosphere, was added n-butyllithium (24.157 ml, 38.651 mmol, 1.6 M in hexanes) dropwise. The resulting reaction mixture was stirred at -78 C for 45 min and then a solution of iodine (9.81 g, 38.651 mmol) in dry THF (20 ml) was added dropwise. The mixture was stirred at -78 C for 1 h, allowed to warm to rt, diluted with EtOAc and quenched with NH4Cl (aqueous sat. solution) and Na2S203 (aqueous sat. solution). The organic layer was separated, washed with NaHC03 (aqueous sat. solution), dried (Na2S04) and concentrated in vacuo. The crude product was purified by column chromatography (silica gel; heptane/DCM up to 20% as eluent). The desired fractions were collected and concentrated in vacuo to yield 1-1 (7.8 g, 81%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping