| 51% |
With 1,1'-bis-(diphenylphosphino)ferrocene; palladium diacetate; triethylamine; In N,N-dimethyl-formamide; at 140℃; under 22502.3 Torr; |
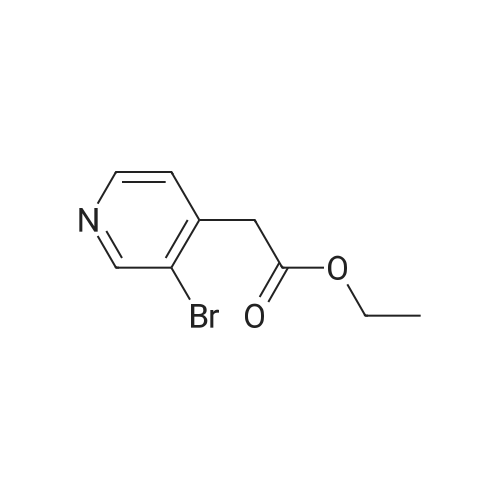
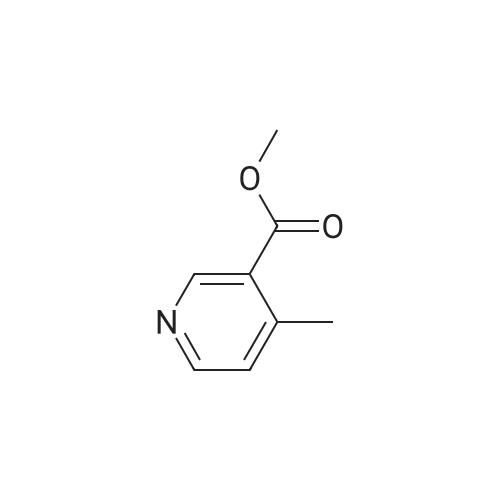
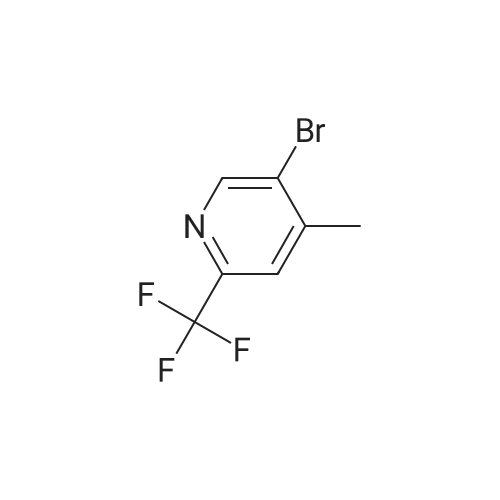
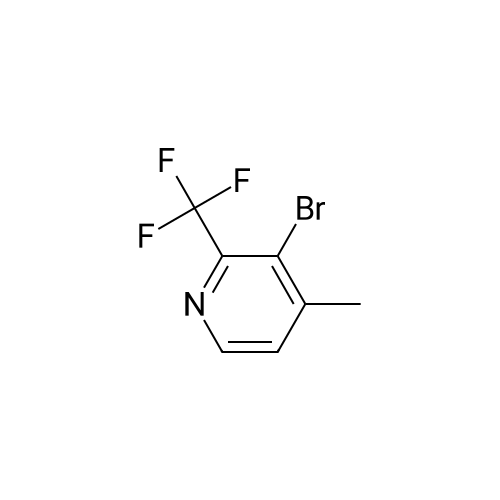
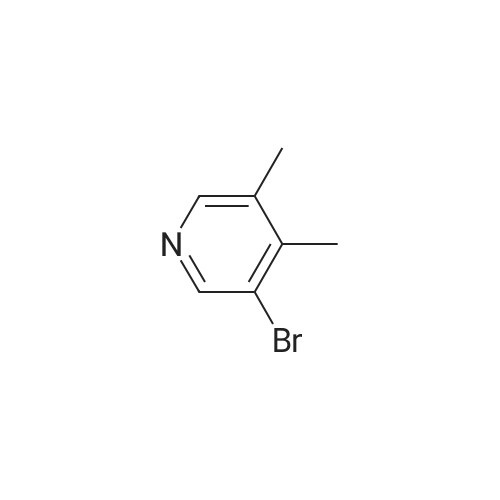
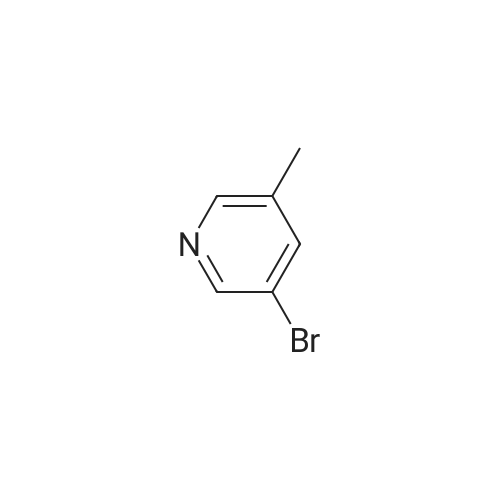
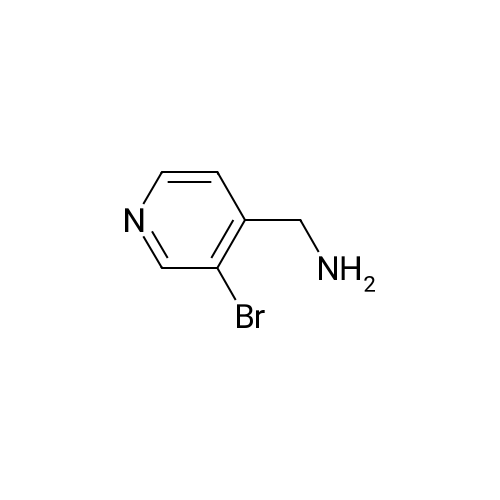
Procedure b: A metal reactor was charged with 3-bromo-4-methyl-pyridine (200 g, 0.116 mol) and a mixture of DMF/MeOH (1 L/1L). To this was added Et3N (400 g, 0.395 mol), palladium (II) acetate (8 g, 0.036 mol) and 1 , l'-bis(diphenylphosphino)- ferrocene (16 g, 0.029 mol). The reactor was closed and pressurized with CO gas (3 MPa) and the reaction mixture was stirred and heated overnight at 140 C. The RM was cooled, filtered and concentrated in vacuo. The residue was purified by flash column chromatography over silica gel (gradient eluent: EtO Ac/Petroleum ether from 1/1 to 1/0). The product fractions were collected and the solvent was evaporated to afford the desired intermediate 1(90 g, 51%). |
| 51% |
With 1,1'-bis-(diphenylphosphino)ferrocene; palladium diacetate; triethylamine; In N,N-dimethyl-formamide; at 140℃; under 22502.3 Torr; |
Procedure b: A metal reactor was charged with 3-bromo-4-methyl-pyridine (200 g, 0.1 16 mol) and a mixture of DMF/MeOH (1 L/1L). To this was added Et3N (400 g, 0.395 mol), palladium (II) acetate (8 g, 0.036 mol) and 1 , -bis(diphenylphosphino)ferrocene (16 g, 0.029 mol). The reactor was closed and pressurized with CO gas (3 MP a) and the reaction mixture was stirred and heated overnight at 140 C. The RM was cooled, filtered and concentrated in vacuo. The residue was purified by flash column chromatography over silica gel (gradient eluent: EtO Ac/Petroleum ether from 1/1 to 1/0). The product fractions were collected and the solvent was evaporated to afford the desired intermediate 1 (90 g, 51%). |
| 51% |
With 1,1'-bis-(diphenylphosphino)ferrocene; palladium diacetate; triethylamine; In N,N-dimethyl-formamide; at 140℃; under 22502.3 Torr; |
A metal reactor was charged with 3-bromo-4-methyl-pyridine (200 g,0.116 mol) and a mixture of DMF/MeOH (1 L/1L). To this was added Et3N (400 g,0.395 mol), palladium (II) acetate (8 g, 0.036 mol) and 1,1?-bis(diphenylphosphino)ferrocene (16 g, 0.029 mol). The reactor was closed and pressurized with CO gas (3 MPa) and the reaction mixture was stirred and heated overnight at 140 C. The RM was cooled, filtered and concentrated in vacuo. The residue was purified by flash column chromatography over silica gel (gradient eluent:EtOAc/Petroleum ether from 1/1 to 1/0). The product fractions were collected and the solvent was evaporated to afford the desired intermediate 1 (90 g, 5 1%). |
| 51% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; palladium diacetate; triethylamine; In N,N-dimethyl-formamide; at 140℃; |
Procedure b: A metal reactor was charged with 3-bromo-4-methyl-pyridine (200 g, 0.116 mol) and a mixture of DMF/MeOH (1 L/1L). To this Et3N (400 g, 0.395 mol), palladium (II) acetate (8 g, 0.036 mol) and 1 , -bis(diphenylphosphino)ferrocene (16 g, 0.029 mol) were added. The reactor was closed and pressurized with CO gas (3 MP a) and the reaction mixture was stirred and heated overnight at 140 C. The RM was cooled, filtered and concentrated in vacuo. The residue was purified by flash column chromatography over silica gel (gradient eluent: EtO Ac/Petroleum ether from 1/1 to 1/0). The product fractions were collected and the solvent was evaporated to afford the desired intermediate 21 (90 g, 51%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping