| 91% |
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃; for 6h; |
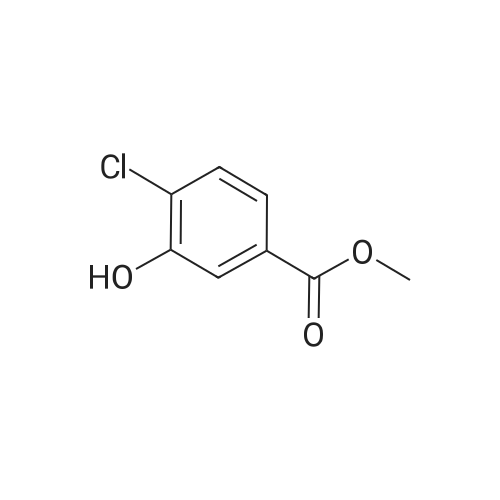
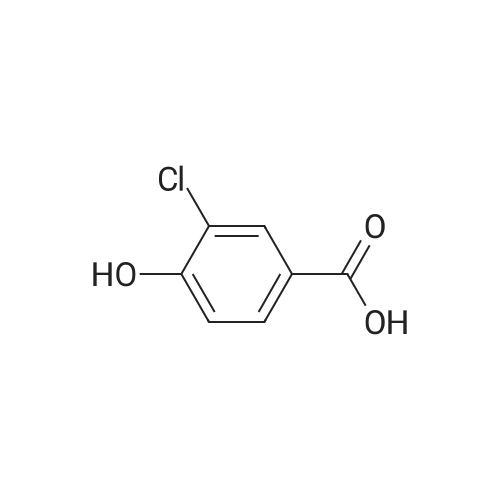
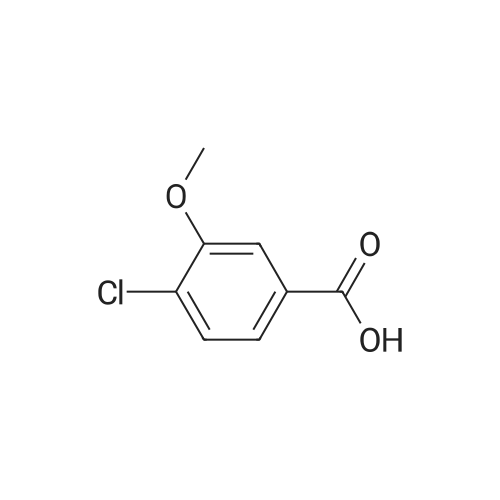
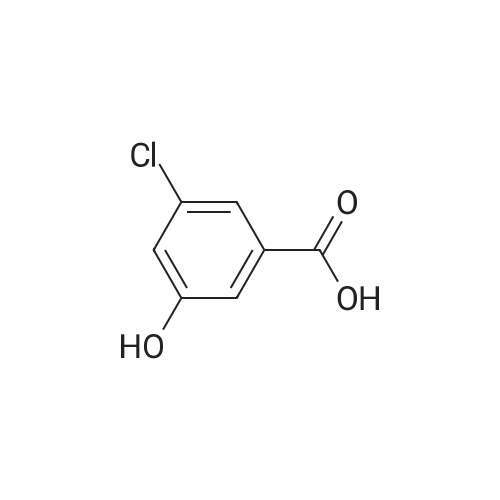
Intermediate D2; 4-Chloro-3-ethoxy-benzaldehvde [CAS RN 85259-46-7]; To a solution of 4-chloro-3-hydroxy-benzoic acid (3.0 g, 17.4 mmol, 1.0 equiv) in DMF (15 mL) was added K2CO3 (4.81 g, 34.8 mmol, 2.0 equiv) and ethyl iodide (4.03 mL, 5.97 g, 38.2 mmol, 2.2 equiv). The reaction mixture was stirred for 6 h at rt, diluted with water (20 mL) and extracted with ethyl acetate (3 x 50 mL). The organic phases were dried over Na2SO4 and concentrated to afford 3.6 g (91%) of 4-chloro-3-ethoxy- benzoic acid ethyl ester. The crude ester was then dissolved in THF (20 mL) and cooled to -78 0C under Ar. A solution of diisobutylaluminium hydride (95 mL, 95.0 mmol, 6.0 equiv; 1.0 M solution in THF) was slowly added over a time period of 15 min, the <n="79"/>cooling bath removed on completion of addition and the reaction allowed to warm up to 0 0C. After stirring for 1 h, the reaction was cooled to -78 0C and the excess hydride quenched by cautious addition of a solution of 1 M HCl (10 mL). The mixture was brought to rt, the organic phase separated and the aqueous layer extracted with ethyl acetate (3 x 100 mL). The combined organic phases were dried over Na2SO4 and concentrated by evaporation under reduced pressure providing 2.94 g (100%) of 4- chloro-3-ethoxy-benzyl alcohol. The crude alcohol (2.94 g, 15.75 mmol, 1.0 equiv) was dissolved in dichloromethane (15 mL) and activated MnO2 (5.48 g, 63.0 mmol, 4.0 equiv) was added. The reaction mixture was stirred for 16 h, after which time the reaction was filtered through Hyflo Super CeI and concentrated. The residue was purified by flash column chromatography on silica eluting with heptane/ ethyl acetate (4:1) to yield 1.51 g (52%) of the title compound. 1H NMR (300 MHz, CDCl3): δ 1.51 ( t, / = 7.1 Hz, 3H), 4.19 (q, / = 7.1 Hz, 2H), 7.37-7.42 (m, 2H), 7.55 (d, / = 9.0 Hz, IH), 9.94 (s, IH). |
| 91% |
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃; for 6h; |
4-Chloro-3-ethoxy-benzaldehyde [CAS RN 85259-46-7] To a solution of 4-chloro-3-hydroxy-benzoic acid (3.0 g, 17.4 mmol, 1.0 equiv) in DMF (15 mL) was added K2CO3 (4.81 g, 34.8 mmol, 2.0 equiv) and ethyl iodide (4.03 mL, 5.97 g, 38.2 mmol, 2.2 equiv). The reaction mixture was stirred for 6 h at rt, diluted with water (20 mL) and extracted with ethyl acetate (3*50 mL). The organic phases were dried over Na2SO4 and concentrated to afford 3.6 g (91%) of 4-chloro-3-ethoxy-benzoic acid ethyl ester. |
| 91% |
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃; for 6h; |
To a solution of 4-chloro-3-hydroxy-benzoic acid (3.0 g, 17.4 mmol, 1.0 equiv) in DMF (15 mL) was added K2CO3 (4.81 g, 34.8 mmol, 2.0 equiv) and ethyl iodide (4.03 mL, 5.97 g, 38.2 mmol, 2.2 equiv). The reaction mixture was stirred for 6 h at rt, diluted with water (20 mL) and extracted with ethyl acetate (3×50 mL). The organic phase was dried over Na2SO4 and concentrated to afford 3.6 g (91%) of 4-chloro-3-ethoxy-benzoic acid ethyl ester. The crude ester was then dissolved in THF (20 mL) and cooled to -78 C. under Ar. A solution of diisobutylaluminum hydride (95 mL, 95.0 mmol, 6.0 equiv; 1.0 M solution in THF) was slowly added over a time period of 15 min, the cooling bath removed after completion of addition and the reaction allowed to reach 0 C. After stirring for 1 h, the reaction was cooled to -78 C. and the excess of hydride quenched by cautious addition of a solution of 1 M HCl (10 mL). The mixture was warmed up to rt, the organic phase separated and the aqueous layer extracted with ethyl acetate (3×100 mL). The combined organic phases were dried over Na2SO4 and concentrated by evaporation under reduced pressure providing 2.94 g (100%) of 4-chloro-3-ethoxy-benzyl alcohol. The crude alcohol (2.94 g, 15.75 mmol, 1.0 equiv) was dissolved in dichloromethane (15 mL) and activated MnO2 (5.48 g, 63.0 mmol, 4.0 equiv) was added. The reaction mixture was stirred for 16 h, after which time the reaction was filtered through Hyflo Super Cel and concentrated. The residue was purified by flash column chromatography on silica eluting with heptane/ethyl acetate (4:1) to yield 1.51 g (52%) of the title compound. 1H NMR (300 MHz, CDCl3): δ 1.51 (t, J=7.1 Hz, 3H), 4.19 (q, J=7.1 Hz, 2H), 7.37-7.42 (m, 2H), 7.55 (d, J=9.0 Hz, 1H), 9.94 (s, 1H). |
| 91% |
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃; for 6h; |
To a solution of 4-chloro-3-hydroxy-benzoic acid (3.0 g, 17.4 mmol, 1.0 equiv) in DMF (15 mL) was added K2CO3 (4.81 g, 34.8 mmol, 2.0 equiv) and ethyl iodide (4.03 mL, 5.97 g, 38.2 mmol, 2.2 equiv). The reaction mixture was stirred for 6 h at rt, diluted with water (20 mL) and extracted with ethyl acetate (3×50 mL). The organic phase was dried over Na2SO4 and concentrated to afford 3.6 g (91%) of 4chloro-3-ethoxy-benzoic acid ethyl ester. The crude ester was then dissolved in THF (20 mL) and cooled to -78 C. under Ar. A solution of diisobutylaluminum hydride (95 mL, 95.0 mmol, 6.0 equiv; 1.0 M solution in THF) was slowly added over a time period of 15 min, the cooling bath removed after completion of addition and the reaction allowed reaching 0 C. After stirring for 1 h, the reaction was cooled to -78 C. and the excess of hydride quenched by cautious addition of a solution of 1 M HCl (10 mL). The mixture was warmned up to rt, the organic phase separated and the aqueous layer extracted with ethyl acetate (3×100 mL). The combined organic phases were dried over Na2SO4 and concentrated by evaporation under reduced pressure providing 2.94 g (100%) of 4-chloro-3-ethoxy-benzyl alcohol. The crude alcohol (2.94 g, 15.75 mmol, 1.0 equiv) was dissolved in dichloromethane (15 mL) and activated MnO2 (5.48 g, 63.0 mmol, 4.0 equiv) was added. The reaction mixture was stirred for 16 h, after which time the reaction was filtered through Hyflo Super Cel and concentrated. The residue was purified by flash column chromatography on silica eluting with heptane/ethyl acetate (4:1) to yield 1.51 g (52%) of the title compound. 1 H NMR (300 MHz, CDCl3): δ 1.51 (t, J=7.1 Hz, 3H), 4.19 (q, J=7.1 Hz, 2H), 7.37-7.42 (m, 2H), 7.55 (d, J=9.0 Hz, 1H), 9.94 (s, 1H). |
| 91% |
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃; for 6h; |
To a solution of 4-chloro-3-hydroxy-benzoic acid (3.0 g, 17.4 mmol, 1.0 equiv) in DMF (15 mL) was added K2CO3 (4.81 g, 34.8 mmol, 2.0 equiv) and ethyl iodide (4.03 mL, 5.97 g, 38.2 mmol, 2.2 equiv). The reaction mixture was stirred for 6 h at rt, diluted with water (20 mL) and extracted with ethyl acetate (3×50 mL). The organic phases were dried over Na2SO4 and concentrated to afford 3.6 g (91%) of 4-chloro-3-ethoxy-benzoic acid ethyl ester. The crude ester was then dissolved in THF (20 mL) and cooled to -78 C. under Ar. A solution of diisobutylaluminium hydride (95 mL, 95.0 mmol, 6.0 equiv; 1 M solution in THF) was slowly added over a time period of 15 min, the cooling bath removed on completion of addition and the reaction allowed to reach 0 C. After 1 h, the reaction was cooled to -78 C. and the excess hydride quenched by cautious addition of a solution of 1 M HCl (10 mL). The mixture was brought to rt, the organic phase separated and the aqueous layer extracted with ethyl acetate (3×100 mL). The combined organic phases were dried over Na2SO4 and concentrated by evaporation under reduced pressure to afford 2.94 g (100%) of 4-chloro-3-ethoxy-benzyl alcohol. The crude alcohol (2.94 g, 15.75 mmol, 1.0 equiv) was dissolved in dichloromethane (15 mL) and activated MnO2 (5.48 g, 63.0 mmol, 4.0 equiv) was added. The reaction mixture was stirred for 16 h, after which time the reaction was filtered through Hyflo Super Cel and concentrated. The residue was purified by flash column chromatography on silica eluting with heptane/ethyl acetate (4:1) to yield 1.51 g (52% yield) of the title compound. 1H NMR (300 MHz, CDCl3): δ 1.51 (t, J=7.1 Hz, 3H), 4.19 (q, J=7.1 Hz, 2H), 7.37-7.42 (m, 2H), 7.55 (d, J=9.0 Hz, 1H), 9.94 (s, 1H). |
| 91% |
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃; for 6h; |
To a solution of 4-chloro-3-hydroxy-benzoic acid (3.0 g, 17.4 mmol, 1.0 equiv) in DMF (15 mL) was added K2CO3 (4.81 g, 34.8 mmol, 2.0 equiv) and ethyl iodide (4.03 mL, 5.97 g, 38.2 mmol, 2.2 equiv). The reaction mixture was stirred for 6 h at rt, diluted with water (20 mL) and extracted with ethyl acetate (3×50 mL). The organic phases were dried over Na2SO4 and concentrated to afford 3.6 g (91%) of 4-chloro-3-ethoxy-benzoic acid ethyl ester. The crude ester was then dissolved in THF (20 mL) and cooled to -78 C. under Ar. A solution of diisobutylaluminium hydride (95 mL, 95.0 mmol, 6.0 equiv; 1.0 M solution in THF) was slowly added over a time period of 15 min, the cooling bath removed on completion of addition and the reaction allowed to reach 0 C. After stirring for 1 h, the reaction was cooled to -78 C. and the excess hydride quenched by cautious addition of a solution of 1 M HCl (10 mL). The mixture was brought to rt, the organic phase separated and the aqueous layer extracted with ethyl acetate (3×100 mL). The combined organic phases were dried over Na2SO4 and concentrated by evaporation under reduced pressure providing 2.94 g (100%) of 4-chloro-3-ethoxy-benzyl alcohol. The crude alcohol (2.94 g, 15.75 mmol, 1.0 equiv) was dissolved in dichloromethane (15 mL) and activated MnO2 (5.48 g, 63.0 mmol, 4.0 equiv) was added. The reaction mixture was stirred for 16 h, after which time the reaction was filtered through Hyflo Super Cel and concentrated. The residue was purified by flash column chromatography on silica eluting with heptane/ethyl acetate (4:1) to yield 1.51 g (52%) of the title compound. 1H NMR (300 MHz, CDCl3): δ1.51 (t, J=7.1 Hz, 3H), 4.19 (q, J=7.1 Hz, 2H), 7.37-7.42 (m, 2H), 7.55 (d, J=9.0 Hz, 1H), 9.94 (s, 1H). |
| 91% |
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃; for 6h; |
Intermediate Bl 4-Chloro-3-ethoxy-benzaldehvde [CAS RN 85259-46-7]. To a solution of 4-chloro-3-hydroxy-benzoic acid (3.0 g, 17.4 mmol, 1.0 equiv) in DMF (15 mL) was added K2CO3 (4.81 g, 34.8 mmol, 2.0 equiv) and ethyl iodide (4.03 mL, 5.97 g, 38.2 mmol, 2.2 equiv). The reaction mixture was stirred for 6 h at rt, diluted with water (20 mL) and extracted with ethyl acetate (3 x 50 mL). The organic phase was dried over Na2SO4 and concentrated to afford 3.6 g (91%) of 4-chloro-3-ethoxy-benzoic acid ethyl ester. The crude ester was then dissolved in THF (20 mL) and cooled to -78 0C under Ar. A solution of diisobutylaluminum hydride (95 mL, 95.0 mmol, 6.0 equiv; 1.0 M solution in THF) was slowly added over a time period of 15 min, the cooling bath removed after completion of addition and the reaction allowed to reach 0 0C. After stirring for 1 h, the reaction was cooled to -78 0C and the excess of hydride quenched by cautious addition of a solution of 1 M HCl ( 10 mL). The mixture was warmed up to rt, the organic phase separated and the aqueous layer extracted with ethyl acetate (3 x 100 mL). The combined organic phases were dried over Na2SO4 and concentrated by evaporation under reduced pressure providing 2.94 g ( 100%) of 4-chloro-3-ethoxy- benzyl alcohol. The crude alcohol (2.94 g, 15.75 mmol, 1.0 equiv) was dissolved in dichloromethane ( 15 mL) and activated Mnθ2 (5.48 g, 63.0 mmol, 4.0 equiv) was added. The reaction mixture was stirred for 16 h, after which time the reaction was filtered through Hyflo Super CeI and concentrated. The residue was purified by flash column chromatography on silica eluting with heptane / ethyl acetate (4:1) to yield 1.51 g (52%) of the title compound. 1H NMR (300 MHz, CDCl3): δ 1.51 ( t, / = 7.1 Hz, 3H), 4.19 (q, / = 7.1 Hz, 2H), 7.37-7.42 (m, 2H), 7.55 (d, / = 9.0 Hz, IH), 9.94 (s, IH). |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃; for 3h; |
To a solution of <strong>[34113-69-4]4-chloro-3-hydroxybenzoic acid</strong> (10 g, 57.9 mmol) in DMF (200 mL) was added iodoethane (20 g, 128.2 mmol) and potassium carbonate (16 g, 115.8 mmol) at room temperature. After 3 hours, the volume was reduced in vacuo to afford a residue, which was dissolved in ethyl acetate (100 mL), washed with brine (4 x 100 mL) and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated in vacuo to afford crude ethyl 4-chloro-3-ethoxybenzoate as a white solid (12 g), which was carried on crude to the next step. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping