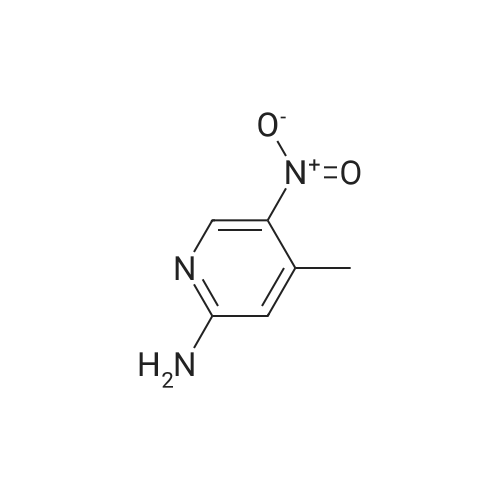
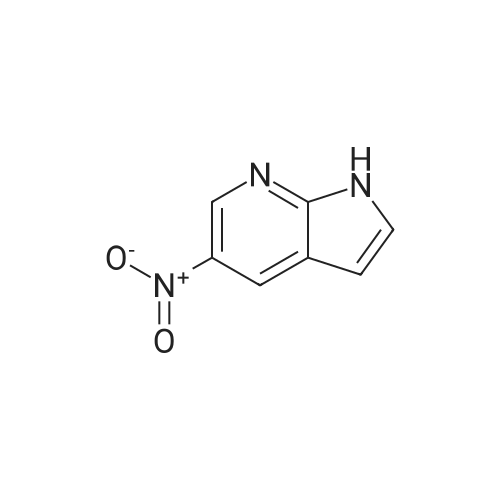
| 88% |
With triethylamine; In isopropyl alcohol; at 90℃; for 0.666667h;Microwave irradiation; |
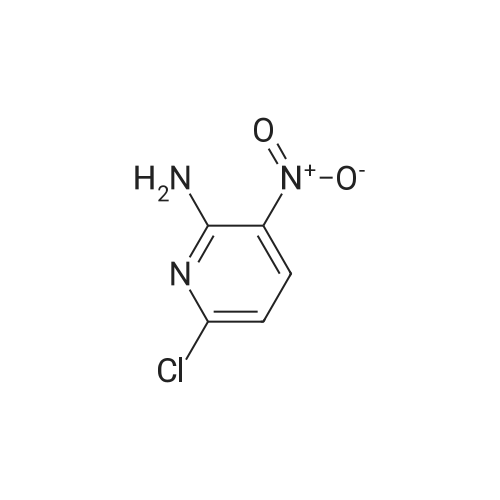
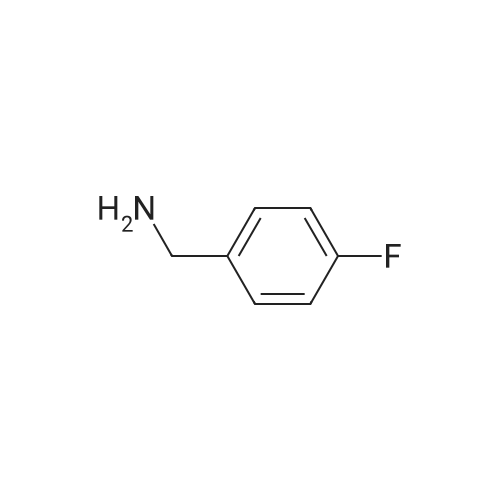
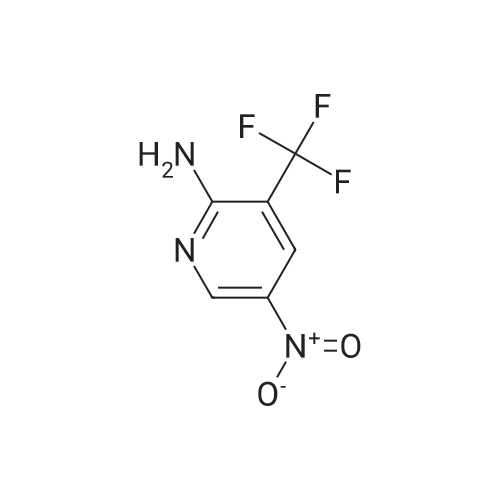
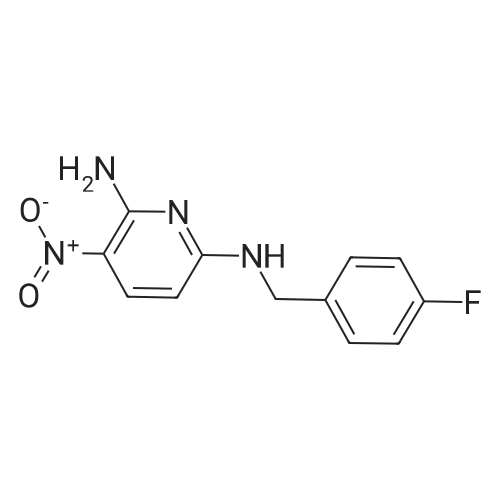
To a solution of Intermediate 5 (500 mg, 2.9 mmol) in isopropanol was added 4- fluorobenzylamine (463 iil, 4.06 mmol) and triethylamine (805 iil, 5.8 mmol). This mixture was stirred at 90C for 40 minutes in the microwave. Water was added to the mixture and the resulting precipitate was filtered off, washed with water and then driedover vacuum for an hour. Intermediate 6 was isolated as a bright yellow solid (661 mg,88%).LCMS (m/z): [IVIH] calcd. for C12H11FN4O2, 262.24; found 263.00. |
| 79.4% |
With triethylamine; In isopropyl alcohol; at 5℃; for 0.5h; |
2-Amino-3-nitro-6-chloropyridine (Compound 1) (50.0 g, 288 mmol, 1.00 eq) was dissolved in isopropyl alcohol (500 mL).p-Fluorobenzylamine (39.7 g, 317 mmol, 1.10 eq) and TEA (32.0 g, 317 mmol, 1.10 eq.Thereafter, the reaction solution was poured into water (1.5 L), and slowly cooled to 5 C and stirred for 30 minutes.Filter and filter cake was washed with water (500 mL).The filter cake was collected and dried to give a yellow solid (Compound 2) 60 g, yield 79.4%. |
| 150 g |
With triethylamine; In water; at 20 - 85℃; |
100 gm of 2-amino-3-nitro-6-chloro-pyridine is taken in 800 ml of water. 90 gm of p-fluorobenzylamine is added dropwise into the reaction mixture at 20-25C. Then 87 gm triethylamine is also added dropwise into the reaction mixture at 20-25C. After complete addition, the reaction mass is stirred at 40-45C for half an hour again the reaction mass is heated to 80-85C and stirred at this temperature for 3-4 hours. After completion of the reaction, the reaction mass is cooled to 20-25C and stirred at this temperature for 2-3 hours and then stirred at 15-20C for 3-4 hours. The solid mass is filtered and then washed with 200 ml of water and 100 ml isopropyl alcohol and then dried in air oven till constant weight to get 140-150 gm. of 2-amino-3-nitro-6-p- fluorobenzylamino-py ridine. |
|
With potassium carbonate; In butan-1-ol; for 2h;Reflux; |
Step-2: Synthesis of Compound 4: 2-Amino-6-chloro-3-nitropyridine 2 (17.35 g, 0.10 mol), 4-fluoro benzyl amine (0.10 mol) and powdered potassium carbonate (10.4 g, 0.035 mol) in n-butanol (100 mL) were heated under reflux for 2 hours. The suspension was filtered and after cooling to room temperature the solid 4 was collected by filtration, washed with butanol, and dried. |
|
at 60 - 65℃; for 6h;Large scale; |
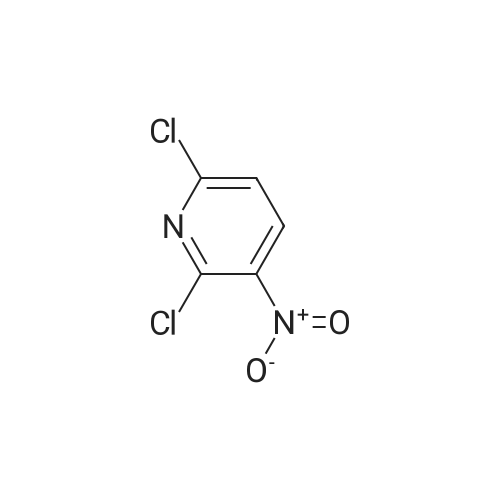
2,6-dichloro-3-nitropyridine (5 kg, 25.9 mol) was added to a reaction vessel containing 20 kg of ethanol, and 25% aqueous ammonia (9.07 kg, 64.8 mol) was added at room temperature to control the internal temperature. 30~35 C, heat preservation reaction for about 6h, TLC monitoring the raw material reaction completely stop the reaction, adding p-fluorobenzylamine (3.8kg, 31.1mol), heating to 60~65 C reaction for about 6h, TLC monitoring the raw material reaction is complete The reaction was stopped, and the temperature was lowered to 5 to 10 C for crystallization for 1 to 2 hours and then filtered. The obtained wet product was added to 60 kg of isopropyl alcohol, heated to reflux, and cooled to room temperature for 2 hours, filtered, and the wet product was kept in the reaction vessel. 75 kg of isopropanol, 5% Pd/C 0.3 kg and ethyl chloroformate (3 kg, 27.6 mol) were added to the reaction vessel, and hydrogen pressure was maintained at 1.2 to 1.5 MPa, and the temperature was controlled at 60 to 70 C for 5 hours. Filtration, the filtrate was cooled to 0~5 C and stirred for 2 h, and dried under reduced pressure at 60 C for 4 h to obtain white flupirtine hydrochloride 6.48 kg, the yield was 73.4%, and the chromatographic purity was 99.62%. The nuclear magnetic data is basically consistent with the data of Example 1. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping