|
|
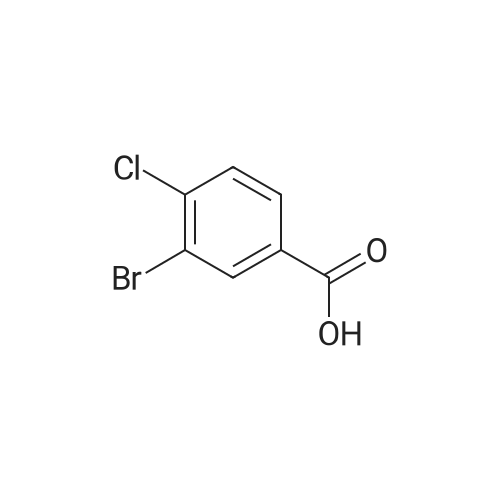
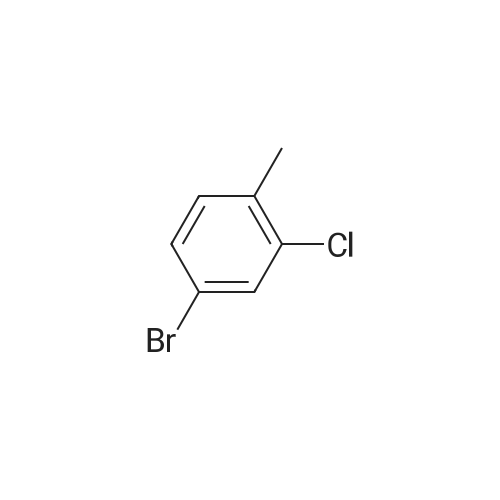
Example 12 4-Aminomethyl-cyclohexanecarboxylic acid {1-[3-(3-amino-1H-indazol-6-yl)-4-chloro-phenyl]-2-phenyl-ethyl}-amide 12A. 3-Bromo-4-chloro-benzaldehyde: To a cooled (0° C.), clear, colorless solution of <strong>[42860-10-6]3-bromo-4-chlorobenzoic acid</strong> (1.0 g, 4.25 mmol) in THF (43 mL) was added dropwise a 1.0 M borane-THF complex (12.7 mL, 12.7 mmol). After 15 min, the reaction was warmed to rt and then to reflux. After 2 h, the reaction was cooled to rt, then to 0° C., and then quenched with MeOH (10 mL). The reaction was warmed to rt and after 15 min., the reaction was concentrated. The residue was dissolved in EtOAc and washed with 1.0 N HCl, sat. NaHCO3, brine, dried over Na2SO4, filtered and concentrated to give a clear, colorless liquid. The liquid was dissolved in CH2Cl2 (17 mL) and cooled to 0° C. Next Dess-Martin periodinane (2.16 g, 5.10 mmol) was added. The resulting cloudy pale orange suspension was stirred for 30 min. and then diluted with Et2O (50 mL). The reaction was filtered through a plug of silica gel and the filtrate was concentrated to give an off-white solid. Column chromatography on silica gel (gradient elution 0-25percent EtOAc in Hex) gave 0.815 g (87percent) of the aldehyde as a white solid. 1H NMR (400 MHz, CDCl3) delta: 9.94 (s, 1H), 8.13 (d, J=1.8 Hz, 1H), 7.77 (dd, J=8.1, 2.0 Hz, 1H), 7.63 (d, J=7.9 Hz, 1H). |
|
With borane; In tetrahydrofuran; at 65℃; for 12h; |
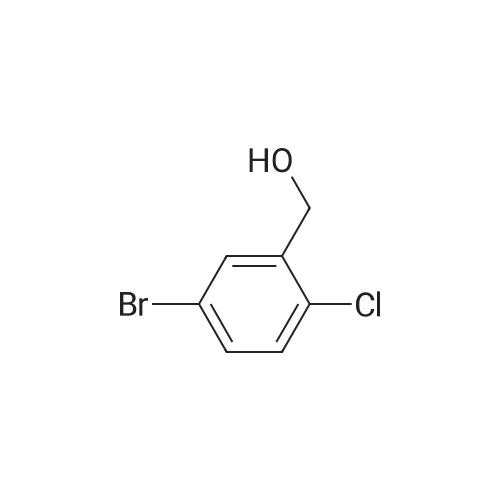
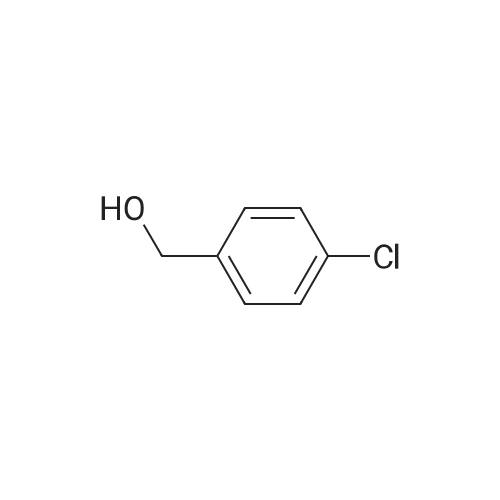
A solution of <strong>[42860-10-6]3-bromo-4-chlorobenzoic acid</strong> (3.53 g, 15 mmol) was dissolved in anhydrous THF (20 [ML)] and cooled to 0 C prior to addition of borane [(1M] soln in [THF,] 20 mL, 20 mmol). The solution was then heated at 65 C for 12 hours then cooled to 0 C and methanol was added dropwise to quench excess borane. The solvent was evaporated under reduced pressure, the residue was redissolved in ethyl acetate and washed with saturated NH4Cl then brine, dried over sodium sulphate. The solvent was removed in vacuo to afford 3.23 g of title compound. [ <P>C7H6BRC10] Mass (calculated): [221], [MH NOT] found. NMR (400 MHz, CDC13) : 4.6 (2H, s, CH20H); 7.15 [(1H,] dd, [J=] 1 and 8 Hz, aryl- H); 7.35 [(1H,] d, [J =] 8 Hz, aryl-H); 7.55 [(1H,] d, [J = 1 HZ,] aryl-H). |
|
|
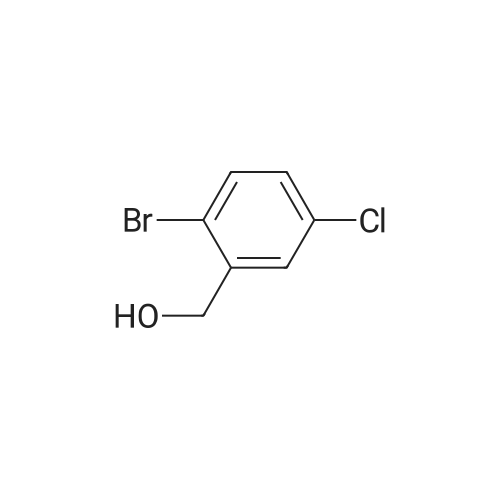
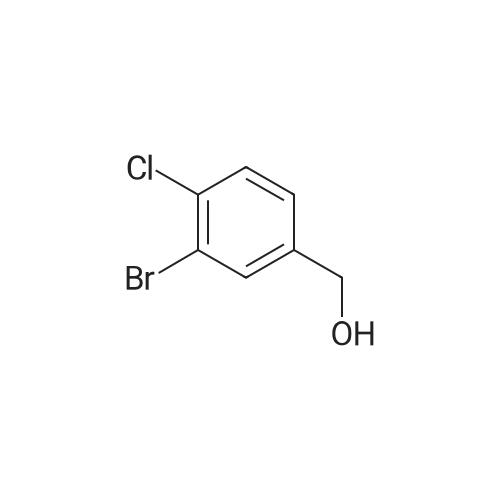
Step 1:199.0 g (0.845 mol) of <strong>[42860-10-6]3-bromo-4-chlorobenzoic acid</strong> were dissolved in 2.5 litres of THF, the mixture was cooled to -10° C. and 1.69 litres (1.69 mol) of a 1 M solution of borane in THF were added at this temperature. The reaction mixture was warmed to RT overnight, and saturated aqueous ammonium chloride solution was then added. After the addition of water, the mixture was extracted twice with ethyl acetate and the combined organic phases were dried over magnesium sulphate and concentrated under reduced pressure. This gave, as a crude product, 206 g of (3-bromo-4-chlorophenyl)methanol which were used in the subsequent step without further purification. |
|
|
Example 28A2-Bromo-4-(bromomethyl)-1-chlorobenzene Step 1:199.0 g (0.845 mol) of <strong>[42860-10-6]3-bromo-4-chlorobenzoic acid</strong> were dissolved in 2.5 litres of THF, the mixture was cooled to -10° C. and 1.69 litres (1.69 mol) of a 1 M solution of borane in THF were added at this temperature. The reaction mixture was warmed to RT overnight, and saturated aqueous ammonium chloride solution was then added. After addition of water, the mixture was extracted twice with ethyl acetate. The combined organic phases were dried over magnesium sulphate and concentrated under reduced pressure. This gave 206 g of (3-bromo-4-chlorophenyl)methanol as a crude product which was reacted without further purification. |
|
With borane-THF; In tetrahydrofuran; at -10 - 20℃; |
Step 1: [0430] 199.0 g (0.845 mol) of <strong>[42860-10-6]3-bromo-4-chlorobenzoic acid</strong> were dissolved in 2.5 litres of THF, the solution was cooled to ?10° C. and 1.69 litres (1.69 mol) of a 1 M solution of borane in THF were added at this temperature. The reaction mixture was warmed to RT overnight, and saturated aqueous ammonium chloride solution was then added. After addition of water, the mixture was extracted twice with ethyl acetate, and the combined organic phases were dried over magnesium sulphate and concentrated under reduced pressure. This gave, as a crude product, 206 g of (3-bromo-4-chlorophenyl)methanol which were used without further purification for the next step. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping