|
With potassium carbonate; In tetrahydrofuran; water; at 0 - 20℃; for 16h; |
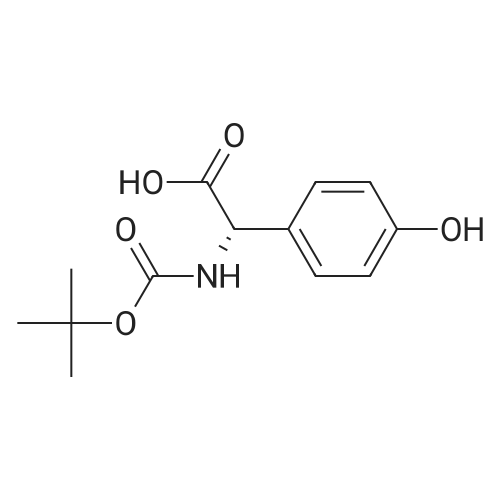
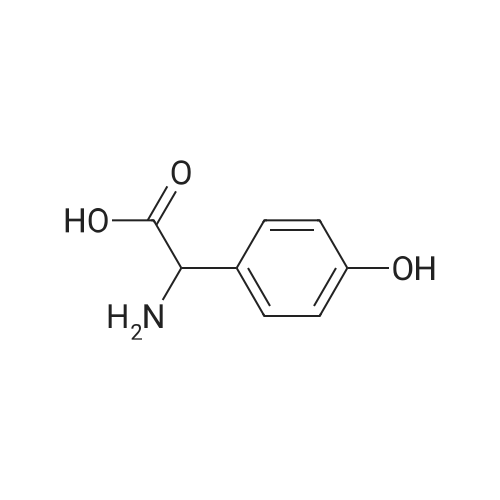
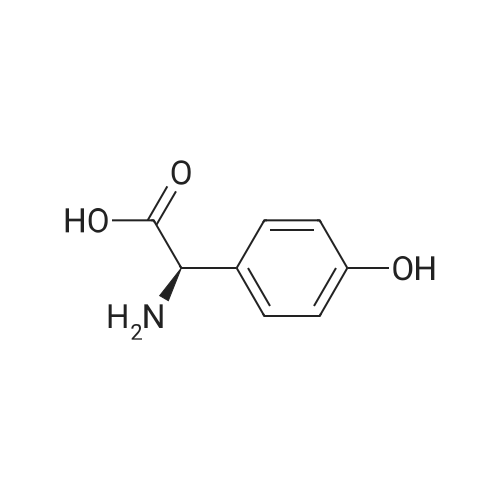
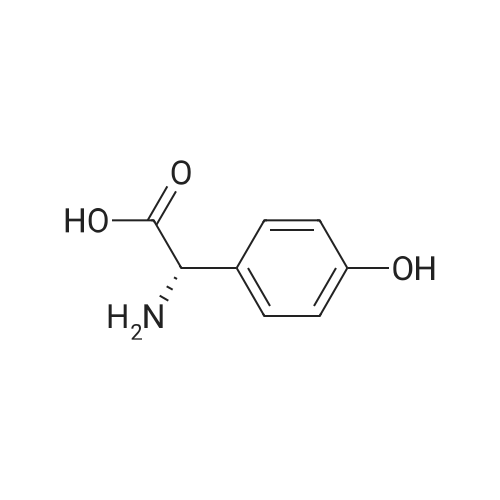
Preparation 14: (.S)-ferf-Butoxycarbonylamino-(4-hydroxyphenyl)acetic acid; (5)-4-Hydroxyphenylglycine (10.00 g, 59.8 mmol) was added to H2O (50 mL) and THF (50 mL) at O0C under argon. K2CO3 (16.40 g, 119.8 mmol) and di-ferf-butyl dicarbonate (14.4 g, 66.0 mmol) were added and the reaction was stirred at ambient temperature for 16 h. The THF was removed in vacuo, then the aqueous layer was extracted with EtOAc (50 mL), acidified to pH 4 with citric acid and further extracted with EtOAc (2 X 50 mL). The combined organic extracts were dried (MgSO4), filtered and concentrated in vacuo, azeotroping several times with DCM to afford the title compound: RT = 2.55 min, m/z (ES+) = 268.1 [M + H]+. |
|
With sodium hydrogencarbonate; In water; acetone; |
To a solution of 4-hydroxyphenylglycine (12 g, 71.860 mmol) in a 1:1 mixture of acetone and water was addeddi-tert-butyldicarbonate (16.5 mL, 71.8 mmol, 1 eq) andsodium bicarbonate (6.03 g, 0.11 mol, 1.5 eq). The solutionwas allowed to stir overnight, and then was quenched with theaddition of citric acid (pH 3) to pH 4. The aqueous layer was65 then extracted 2x with EtOAc and the combined organiclayers were washed with brine, dried over Na2S04 and concentratedto a white foam. The crude material (18.43 g, 69mmol (assumed)) was used without further purification bydissolving it in anhydrous DMF and treating sequentiallywith triethylamine (12.6 mL, 75.9 mmol, 1.3 eq), HOBT(9.32 g, 69 mmol, 1 eq) andAla-OMe HCl (9.63 g, 69 mmol,1 eq). The solution was then cooled to oo C. and EDC (19.55 5g, 0.1 mol, 1.5 eq) was added in one portion. The reaction wasallowed to warm to room temperature and stirred overnight.Water and EtOAc were added, the aqueous layer wasextracted 3x, and the combined organic layers were washedwith brine, dried over Na2S04 and concentrated. The residue 10was purified by flash chromatography (6% MeOH, 0.6%AcOH in DCM) to give a clear residue (17.82 g, 71% yield).Rf=0.39 (7% MeOH in DCM). 1H NMR (CDC13 , 600 MHz)o (ppm) 7.11 (d, 1=8.4 Hz, 2H), 6.64 (d, 1=8.4 Hz, 2H), 6.51 15(brd, 1=6.6 Hz, lH), 5.71 (brs, lH), 5.07 (brs, lH), 4.57-4.52(m, 1H),3.69(s,3H), 1.42-1.40(m, 12H). 13CNMR(CDC13 ,600 MHz) o (ppm) 173.2, 170.5, 156.6, 155.4, 129.0, 128.7(2C), 116.1 (2C), 80.5, 58.2, 52.7, 48.5, 28.4 (3C), 18.4. IR(film)vmax=1655, 1512,1450,1365,1215,1157,1049 cm-1. 20ESI HRMS calcd for [(M+NatJ C17H24N20 6 : 375.1526.found: 375.1532. |
|
With sodium hydrogencarbonate; In water; acetone; at 25℃; |
To a stirred mixture of (S)-2-amino-2-(4-hydroxyphenyl)acetic acid (100 g, 0.6 mol, 1 eq) in a mixture of acetone (400 mL) and water (400 mL) was added di-tert-butyl dicarbonate (130.5 g, 0.6 mol, 1 eq) and NaHCO3 (75.4 g, 0.9 mol, 1.5 eq). The mixture wasallowed to stir at 25 C overnight. After HPLC showed the reaction was complete, the mixture was acidified with 5% citric acid (pH 3). The mixture was filtered and the filter cake was washed with water, then dried to give (S)-2-((tert-butoxycarbonyl)amino)-2-(4- hydroxyphenyl)acetic acid (140 g, 87.5%). The crude product was used directly without further purification. |
|
With sodium hydrogencarbonate; In water; acetone; at 25℃; |
To a stirred mixture of (S)-2-amino-2-(4-hydroxyphenyl)acetic acid (100 g, 0.6 mol, 1 eq) in a mixture of acetone (400 mL) and water (400 mL) was added di-tert-butyl dicarbonate (130.5 g, 0.6 mol, 1 eq) and NaHCO3 (75.4 g, 0.9 mol, 1.5 eq). The mixture wasallowed to stir at 25C overnight. After HPLC showed the reaction was complete, the mixture was acidified with 5% citric acid (pH 3). The mixture was filtered and the filter cake was washed with water, then dried to give (S)-2-((tert-butoxycarbonyl)amino)-2-(4- hydroxyphenyl)acetic acid (140 g, 87.5%). The crude product was used directly without further purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping