| 48% |
|
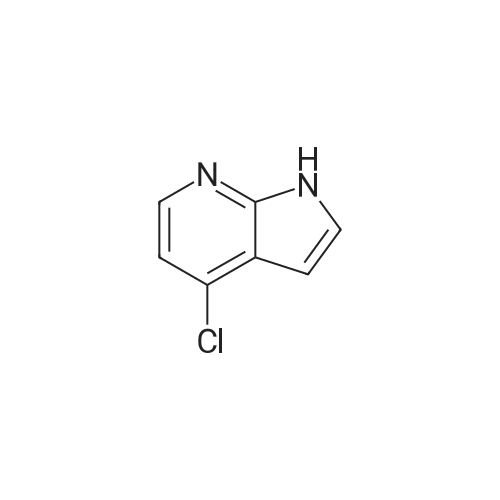
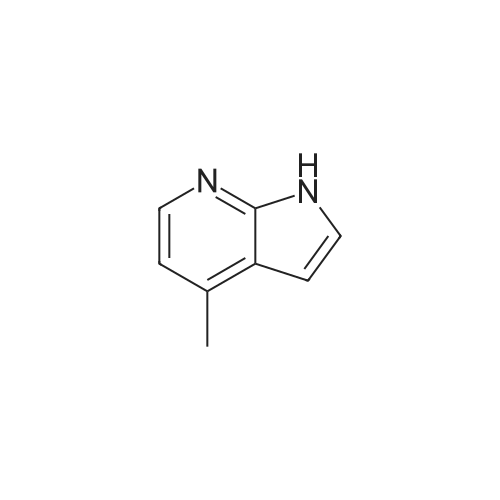
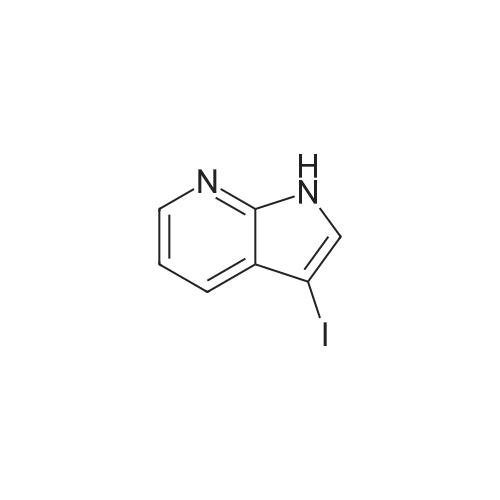
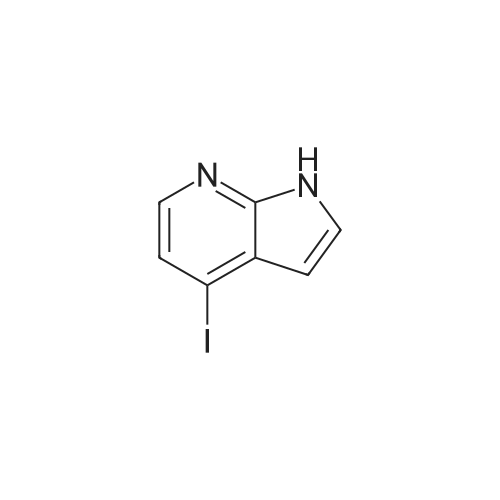
Part 2: [0195] 4-Iodo-1H-pyrrolo[2,3-b]pyridine: To a solution of 4-Chloro-1H-pyrrolo[2,3-b]pyridine (12.9 g, 84.3 mmol) and NaI (40 g, 168 mmol) in acetonitrile (150 mL) was slowly added acetyl chloride (12.6 mL, 176 mmol). The mixture was allowed to stir at 80 C. for 4 days, and then the excess acetonitrile was removed in vacuo. 300 mL of 10% K2CO3 (aq) was added to the residue and the mixture extracted with CH2Cl2 (3x100 mL). The combined organic extracts were washed with 10% sodium bisulfite (aq) and brine, dried over anhydrous sodium sulfate and concentrated in vacuo to give crude product (22.2 g). To a solution of this crude product in THF (150 mL) was added 1M NaOH (100 mL). The mixture was stirred at room temperature for 2 hr prior to evaporation of the solvent in vacuo, dilution with water and extraction with CH2Cl2. The extracts were washed with brine, dried over anhydrous sodium sulfate and concentrated in vacuo. The resulting brown solid was purified by chromatography over silica gel and recrystallized from acetonitrile to give pure 4-Iodo-1H-pyrrolo[2,3-b]pyridine (9.75 g, 48%). MS (ES+): 245 [MH+]. |
| 39% |
|
Intermediate 1.1: 4-iodo-1H-pyrrolo[2,3-b]pyridine Acetyl chloride (2.34 mL, 2.57 g, 32.8 mmol) was added dropwise to a solution of 4-chloro-1H-pyrrolo[2,3-b]pyridine (2.00 g, 13.1 mmol) and sodium iodide (13.8 g, 91.8 mmol) in acetonitrile (25 mL). The resulting suspension was heated at 80 C. for 7 days. After cooling to room temperature, the reaction mixture was concentrated under vacuo, and a saturated aqueous solution of potassium carbonate (50 mL) was added to the residue. The mixture was then extracted with dichloromethane (3*50 mL), the combined organic phase was washed with a saturated solution of sodium bisulfite (2*50 mL) and brine (50 mL), dried over sodium sulfate and concentrated under vacuo. The residue was dissolved in THF (25 mL) and added to an aqueous solution 1N of sodium hydroxide (15 mL). The resulting solution was stirred at 25 C. for 3 h. The reaction mixture was then concentrated under vacuo, and water (100 mL) was added to the residue. The mixture was extracted with dichloromethane (3*50 mL), the combined organic phase was washed with brine (50 mL), dried over sodium sulfate and concentrated under vacuo. The residue was purified by chromatography on a SP1 Biotage system, using hexanes and ethyl acetate as eluents to afford the title compound (1.26 g, 39%) as a yellow solid (HPLC: 66%, RT: 5.77 min) 1H NMR (CDCl3) delta=11.77 (br s, 1H), 7.94 (d, J=5.1 Hz, 1H), 7.51 (d, J=5.1 Hz, 1H), 7.44 (d, J=3.7 Hz, 1H), 6.41 (d, J=3.3 Hz, 1H); MS (m/z) 245 [M+H]+ (127I). |
| 39% |
With acetyl chloride; sodium iodide; In acetonitrile; at 80℃; for 168h; |
Step 1 : To a solution of 4-chloro-1 H-pyrrolo[2,3-b]pyridine (2.00 g, 13.1 mmol) and sodium iodide (13.8 g, 91.8 mmol) in acetonitrile (25 mL) was added dropwise acetyl chloride (2.34 mL, 2.57 g, 32.8 mmol) and the resulting suspension was heated at 80 C for 7 days. After cooling the reaction mixture was concentrated under vacuo and a saturated aqueous solution of potassium carbonate (50 mL) was added to the residue. The mixture was extracted with dichloromethane (3 x 50 mL), the combined organic phase washed with a saturated solution of sodium bisulfite (2 x 50 mL) and brine (50 mL), dried over anhydrous sodium sulfate, filtered and concentrated under vacuo. To a solution of the residue in THF (25 mL) was added an aqueous solution 1 N of sodium hydroxide (15 mL) and the resulting solution was stirred at room temperature for 3 h. The reaction mixture was then concentrated under vacuo and water (100 mL) was added to the residue. The mixture was extracted with dichloromethane (3 x 50 mL), the combined organic phase was washed with brine (50 mL), dried over anhydrous sodium sulfate, filtered and concentrated under vacuo. The residue was purified on silica gel column using column using hexane / ethyl acetate as eluent to give 4-iodo- H-pyrrolo[2,3- b]pyridine (1.26 g, 39%) as a yellow solid; LCMS (ESI) 245 (M+H); HPLC 66%, RT: 5.77 min; H NMR (400 MHz, CHLOROFORM-d) delta ppm: 1 1.77 (br s, 1 H), 7.94 (d, J=5.1 Hz, 1 H), 7.51 (d, J=5.1 Hz, H), 7.44 (d, J=3.7 Hz, 1 H), 6.41 (d, J=3.3 Hz, 1 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping