| 70% |
|
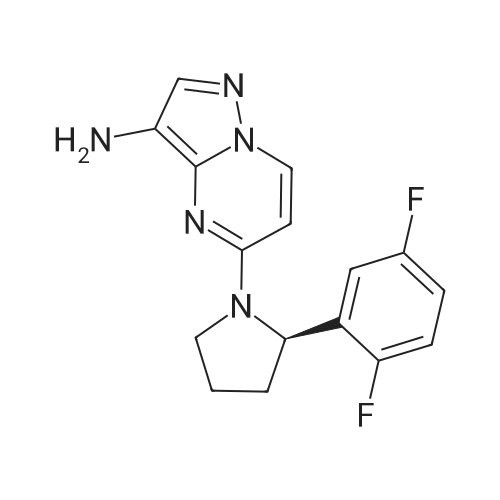
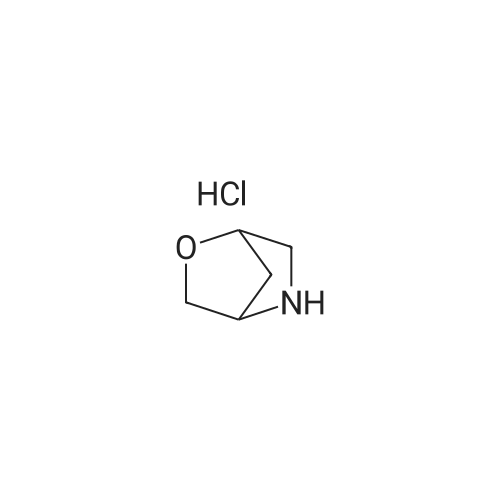
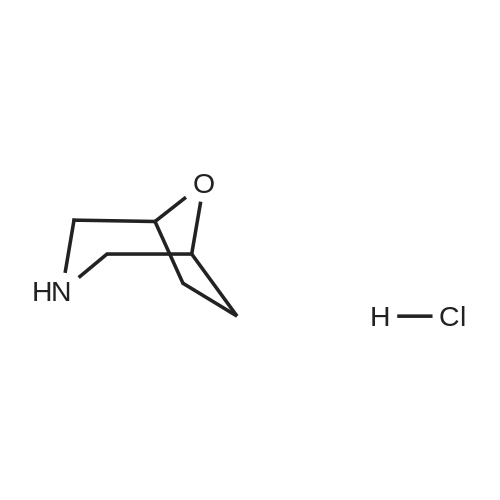
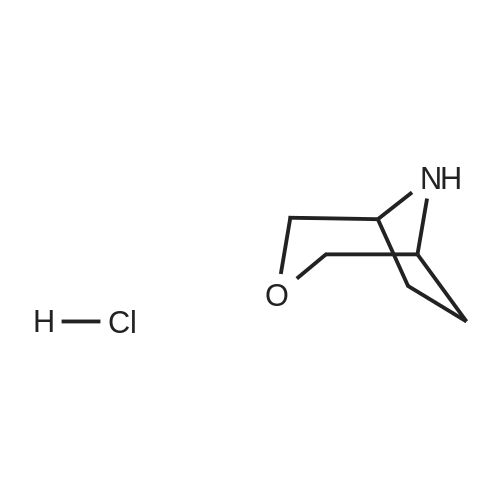
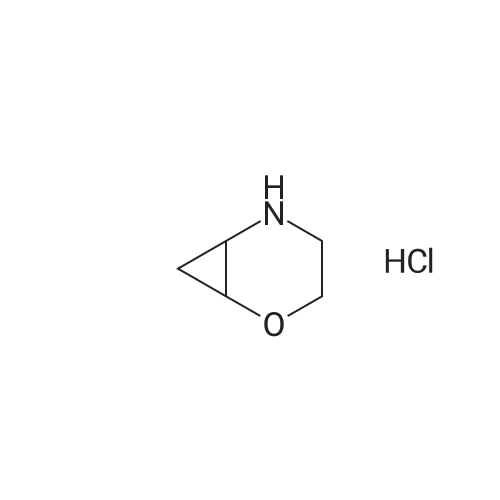
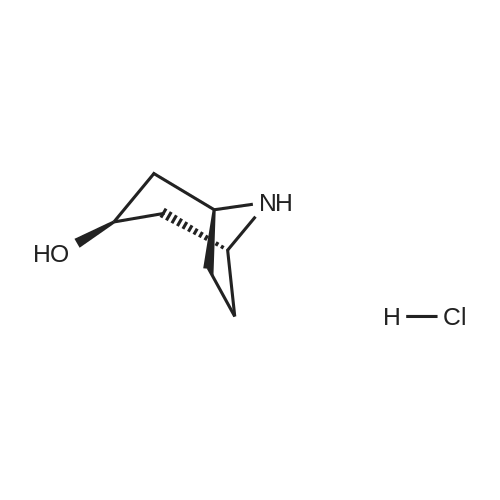
Example 1; 9beta-13-O-[(2R,3S)-3-(tert-butyloxycarbonylamino)-2-hydroxyl-3-phenyl propionyl]-10-deacetyl-9-dihydro-9,10-O-[2-N-(2S,4S)-2-oxa-5-aza-bicyclo[2.2.1 ] hept-5-ylmethyl] baccatinIII ; [Show Image] The resulting compound from step 7, 9beta-13-O-[(2R,3S)-3-(tert-butyloxycarbonyl amino)-2-hydroxyl-3-phenylpropionyl]-10-deacetyl-9-dihydro-9,10-O-acetaldehyde baccatin H(0.545g, 0.63mmol, 1.0eq) was dissolved in 30ml anhydrous methanol in a 100ml three-neck flask to form a solution under an argon atmosphere. And then, a minor amount of drying molecular sieve (4A) and (S,S)-2-oxa-5-aza-bicyclo[2.2.1] heptane hydrochloride (0.568g, 4.16mmol, 6.6eq) were added into the solution during stirring at room temperature to form a mixture. Upon completion of the addition, the mixture was stirred for 30 minutes at room temperature. Sodium cyanoborohydride (0.261g, 4.16mmol, 6.6eq) was added into the mixture. Upon completion of the addition, the mixture was stirred for 1.5 hours at room temperature. It is shown that the starting materials are completely reacted during the reaction according to the Point-plate tracking (dichloromethane: ethyl acetate: methanol = 10:10:1). The resulting mixture was quenched with 70ml saturated sodium bicarbonate solution, extracted with ethyl acetate (100 ml.x.4) to form the organic extracts. The combined organic extracts were washed with 25ml water and 25ml saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to form a residue. The residue was purified by silica gel column chromatography with hexane: dichloromethane: ethyl acetate: methanol =20:10:10:2 as eluents firstly, then with dichloromethane: ethyl acetate: methanol =30:10:2 to provide 9beta-13-O-[(2R,3S)-3-(tert-butyloxycarbonylamino)-2-hydroxyl-3-phenylpropionyl] -10-deacetyl-9-dihydro-9,10-O-[2-N-(2S,4S)-2-Oxa-5-aza-bicyclo[2.2.1]hept-5-ylm ethyl] baccatinIII I (0.412 g, white-like solid) with a yield of 70percent. Rf=0.29(dichloromethane: ethyl acetate: methanol = 10:10:1(V/V)) MW=933, ESI-MS:[M+H]+=933.9. 1H-NMR(CD3Cl3, 400MHz): delta8.1(d, J=7.5Hz, 2H, Ar-H), 7.7-7.5(t, J=7.0Hz, 1H, Ar-H), 7.5-7.2(m, 7H), 6.1-6.0(m, 2H), 5.7-5.6(d, J=9.4Hz, 1H), 5.3(d, J=9.4Hz, 1H), 5.2(d, J=7.0Hz, 1H), 5.1(s, 1H), 5.0-4.9(b, 1H), 4.7-4.6(d, J=8.2Hz, 1H), 4.6(b, 1H), 4.4(b, 1H), 4.4-4.2(dd, AB-type, J=8.4Hz, 2H), 4.2-4.0(m, 2H), 4.0(d, J=7.9Hz, 1H), 3.8(d, J=7.1Hz, 1H), 3.7-3.6(d, J=7.8Hz, 1H), 3.6(b, 1H), 3.1-3.0(m, 2H), 3.0-2.9(m, 1H), 2.9(d, J=4.7Hz, 1H), 2.7(m, J=10.2Hz, 1H), 2.4(dd, J=9.7Hz, J'=14.6Hz, 1H), 2.3(s, 3H, CH3), 2.3-2.2(m, 1H), 2.2-2.0(m, 2H), 1.9(b, 1H), 1.9-1.8(m, 1H), 1.8-1.7(m, 1H), 1.65(s, 3H, CH3), 1.60(s, 3H, CH3), 1.55(s, 3H, CH3), 1.4(s, 9H, t-Bu), 1.3(s, 3H, CH3). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping