|
With potassium carbonate; In dimethyl sulfoxide; at 120℃; for 17h; |
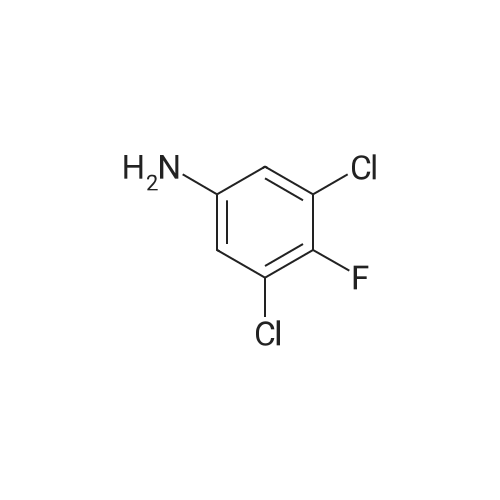
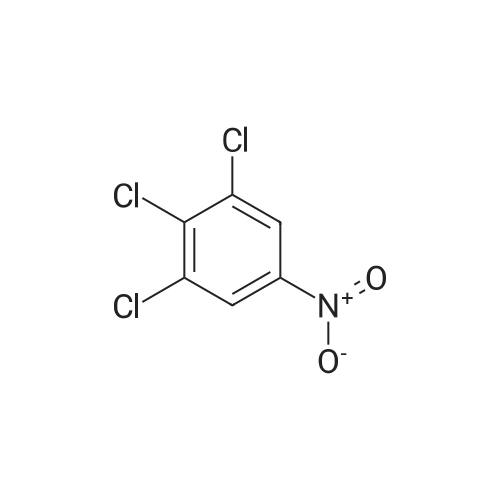
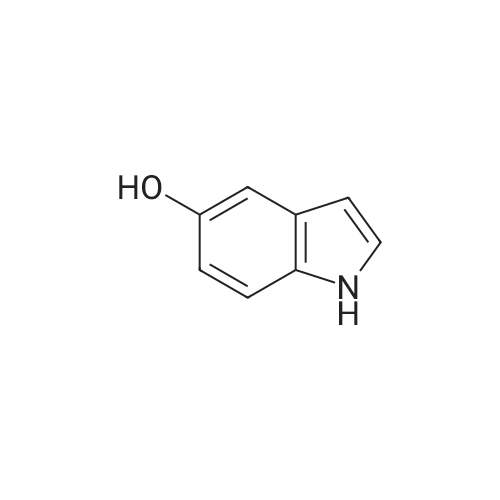
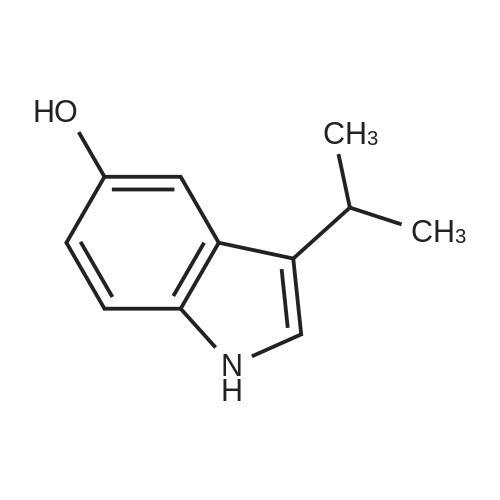
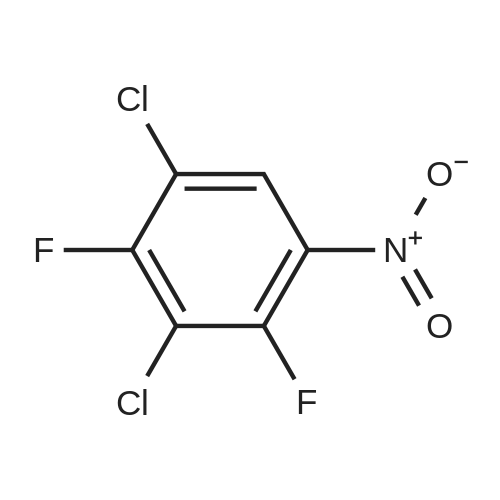
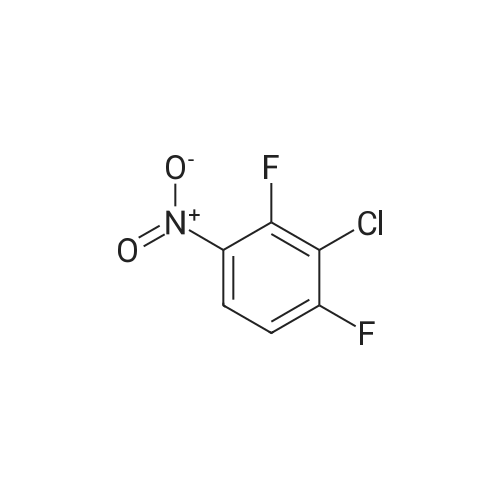
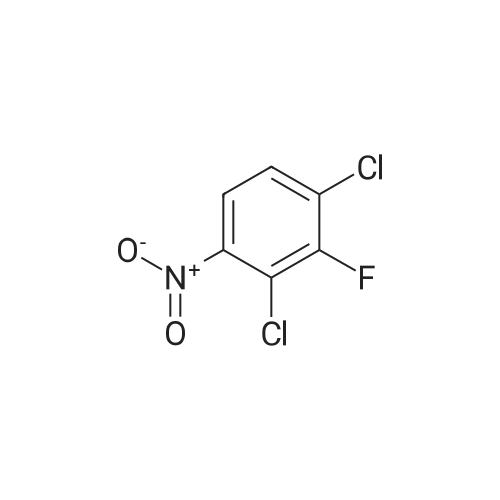
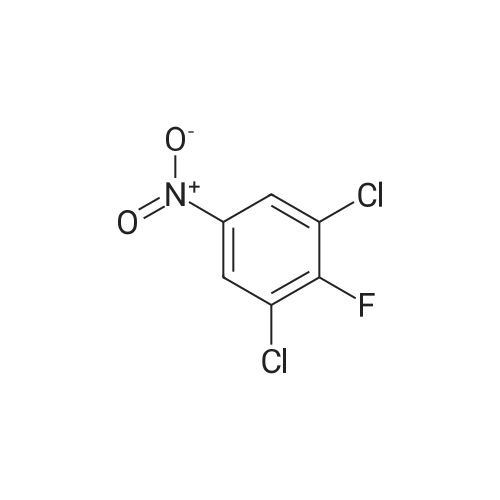
To a stirred solution of the 5-Methoxy-3-methyl-lH-indole-2-carboxylic acid (42 mg, 0.125 mmol), made by basic hydrolysis from the commercially available 5-Methoxy-3-methyl-l H-indole-2- carboxylic acid ethyl ester (purchased from Zerenex Molecular Ltd, UK), in DMF (3 ml) was successively added 1-hydroxybenzotriazole hydrate (60 mg, 0390mmol) and N,N-l -(3- dimethylaminopropyl)-3-ethyl-carbodiimide hydrochloridethyl (75 mg, 0.390mmol) and isopropylamine( 40 mg, 0.39 mmol) at 0 0C. After being stirred for 1 h at 0 C the mixture was allowed to reach ambient temperature and was left stirring for 17 h.After filtration of the mixture, the filtrate was evaporated in vacuo. Purification on a silica SPE column (heptane:EtOAc 7:3) gave 5-methoxy-3-methyl-lH-indole-2-carboxylic acid <n="40"/>isopropylamide as a solid. The indole was dissolved in dichloromethane (10 mL) which was cooled to 0 0C and put under nitrogen atmosphere. Boron trifluoride dimethyl sulphide complex (3 equivalents) was added. The organic phase was washed with water, brine, dried over Na2SO4, filtrated and evaporated. The crude was utilised without further purification. The crude (30 mg) containing 5-Hydroxy-3-methyl-l H-indole-2-carboxylic acid isopropylamide, l,3-Dichloro-2- fluoro-5-nitro-benzene (22 mg, 0.1 mmol) and K2CO3 (23 mg, 0.17 mmol) was dissolved in DMSO (1.5 mL), purged with N2 and stirred at 120 0C for 17 h. The mixture was diluted with EtOAc and washed with a saturated Na2Ctheta3 solution, water, and brine. The organic phase were evaporated on silica and purified on a silica column (heptane: EtOAc, 7:3) to give 20 mg of 5-(2,6-dichloro-4- nitro-phenoxy)-3-methyl-l H-indole-2-carboxylic acid isopropylamide as a solid.The biaryl indole was dissolved in a mixture of HOAc and H2O (9: 1 , 5 mL) and Fe (27 mg) was added and the reaction was left stirring at ambient temperature for 3 h. The reaction was diluted with EtOAc and HCl (2M) was added. The organic phase was separated and the water phase extracted twice with EtOAc. The combined organic phases were washed with brine, dried with Na2SO4, filtrated and evaporated. The evaporation gave 17 mg as a brown oil which was used without further purification.To the crude (17 mg) containing 5-(4-amino-2,6-dichloro-phenoxy)-3-methyl-lH-indole-2- carboxylic acid isopropylamide, which was dissolved in CH2CI2 (10 mL) and cooled to 0 C, was added ethyl malonyl chloride (1 1 muL, 0.087 mmol) and pyridine (9 muL, 0.1 1 mmol). After 2.5 h at O0C the reaction was quenched by addition of HCl (3 mL, IM). After stirring for 10 min, additional CH2Cl2 (30 mL) and HCl (1 mL, 1 mL) was added. The organic layer was washed with HCl (IM), H2O, brine and evaporated. The crude was dissolved in THF (5 ml) and LiOH (IM, 2 ml) and left overnight. The reaction mixture was acidified to pH 3 with HCl (2M). EtOAc was added and the organic phase was washed with brine, dried with Na2SO4, filtrated and evaporated. The ethyl ester was dissolved in THF (5 ml) and LiOH (IM, 2 ml) was added. The reaction was left stirring for 4 days at ambient temperature. HCI (2M) was added until pH 3 was reached. Extraction with EtOAc (2x20 ml), washed with brine, dried with Na2SO4 and evaporated. The crude was purified by semi-preparative HPLC (Zorbax CombiHT (SB-C8 50x21.2 mm, 5mu) Mobile Phase: Solvent A. Water with 0.5% formic acid; Solvent B: acetonitrile. Gradient: 2 min 80% of A then over 8 min to 5% of A) giving 2.5 mg as a solid (yield: 4.1 %) LCMS. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping