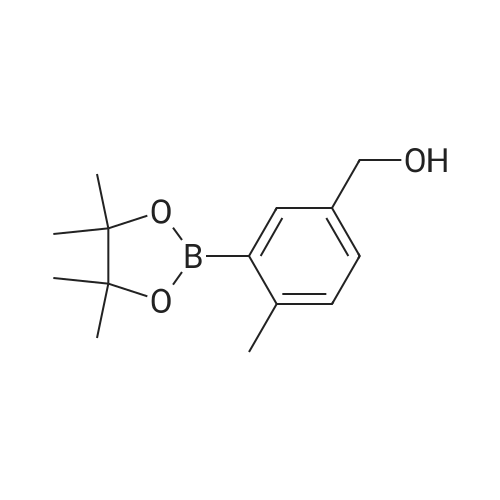
|
With triethylamine; In dichloromethane; at 0℃; for 2h;Inert atmosphere; |
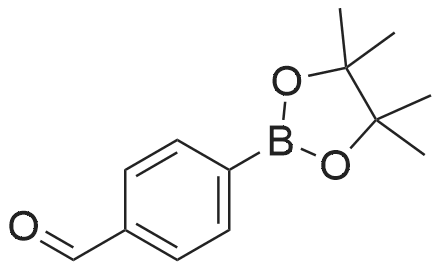
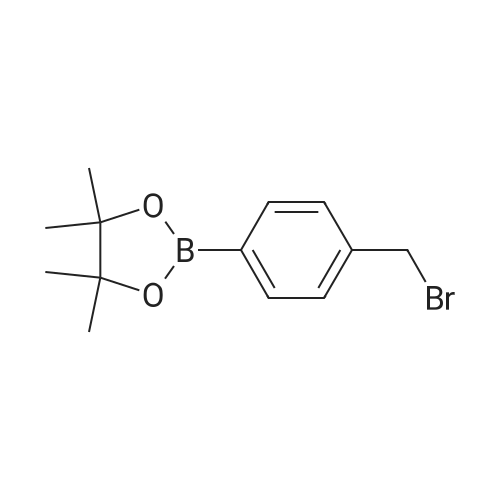
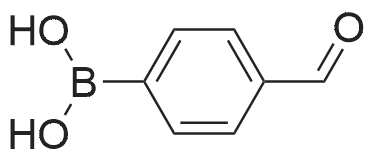
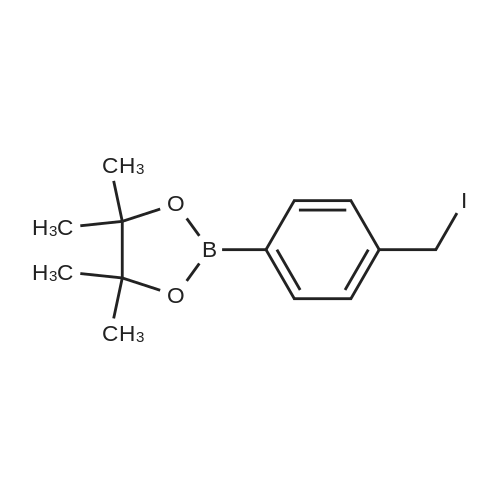
(ii) Synthesis of Compound (15); (4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl methanesulfonate) (see Non-patent Literature 14); Under argon atmosphere, triethylamine (3.15 mL, 22.6 mmol) and methanesulfonyl chloride (1.40 mL, 18.1 mmol) were sequentially added to a CH2Cl2 (60 mL) solution of Compound (14) (3.48 g, 14.9 mmol), and the mixture was stirred at 0 °C for 2 hours. Water (150 mL) was added to the mixture, and the mixture was extracted with CH2Cl2 (100 mL .x. 3). All the organic phases were mixed, sequentially rinsed with water (70 mL .x. 3) and a saline solution (70 mL .x. 3), dried (Na2SO4), filtered, and concentrated under reduced pressure. The residue was purified by flash column chromatography (110 g of silica gel, n-hexane/EtOAc = 3/1) to obtain Compound (15) as a colorless solid. The Compound (15) was not purified furthermore and used in the next step. TLC 0.41 (n-hexane/EtOAc = 2/1). |
|
With N-ethyl-N,N-diisopropylamine; In dichloromethane; at 0℃; for 3h;Inert atmosphere; |
2-[4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl]isoindoline-1,3-dione (3a). Compound 2a (585 mg, 2.50 mmol), and dichloromethane (25.00 mL) were added to a dry flask containing a magnetic stir bar under a nitrogen atmosphere. The flask was cooled to 0 °C, then methanesulfonyl chloroide (0.29 mL, 3.75 mmol) and N,N-diisopropylethylamine (DIPEA, 0.87 mL, 5.00 mmol) were slowly added to the flask. The reaction mixture was stirred at 0 °C for 3 h. After the reaction was completed, the reaction mixture was diluted with dichloromethane (25.00 mL) before H2O (25.00 mL)was added. The organic layer was then washed with brine and dried with MgSO4. The resulting organic layer was then filtered and the filtrate was concentrated in vacuo. The resulting crude material was re-dissolved in DMF (4.68 mL) after which both potassium phthalimide salt (695 mg, 3.75 mmol), and K2CO3 (1,036 mg, 7.50 mmol) were added to the solution. The reaction was then allowed to stir at room temperature for 3 days. After the reaction was completed, the distilled H2O (20.00 mL) was slowly added to the reaction mixture to afford the formation of a solid precipitate. The reaction mixture was then filtered and the filtered cake was collected. The filtered cake was re-dissolved in tertbutanol/H2O (4:1, v/v) (10.00 mL) before a freeze-drying process was applied to remove the remaining DMF. The desired product 3a was obtained as a white solid (m.p. 166?169 °C) in 96percent yield (872 mg);1H-NMR (CDCl3) delta ppm 7.84?7.82 (m, 2 H), 7.74 (d, J = 7.85 Hz, 2 H), 7.71?7.68 (m, 2H), 7.42 (d,J = 7.85 Hz, 2H), 4.85 (s, 2H), 1.34 (s, 12H); 13C-NMR (CDCl3) delta ppm 167.90, 139.26, 135.12,133.93, 132.04, 127.76, 123.29, 83.73, 41.60, 24.77; 11B-NMR (CDCl3) delta ppm 30.94. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping