| 96.8% |
With phosphorus pentachloride; trichlorophosphate; at 5 - 100℃; for 7h; |
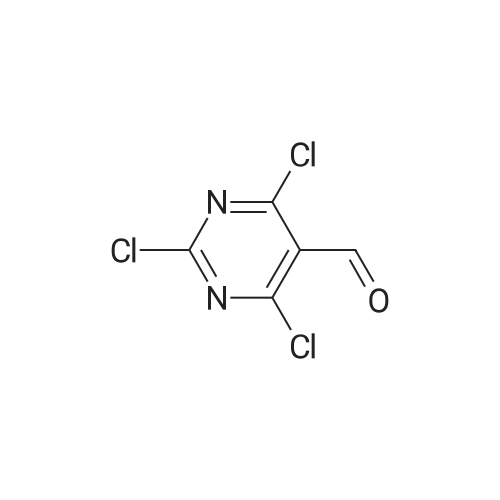
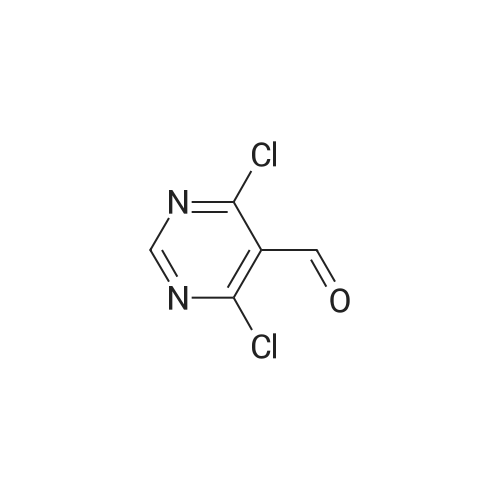
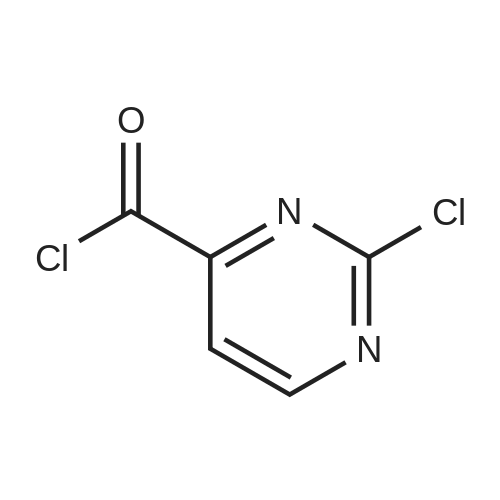
(1) Firstly disperse 125 g of uracil-5-carboxylic acid in 400 ml of phosphorus oxychloride, cool to 5-10 C, add 500 g of phosphorus pentachloride to complete mixing, and heat to 100 C for reflux reaction, the reaction time is 7 hours;(2) After the reflux reaction is completed, the temperature is reduced, and excess phosphorus oxychloride is removed by a reduced pressure distillation method;(3) 177 g of pure product was obtained by distillation under reduced pressure, the purity was greater than 97%, and the yield was 96.8%. |
| 96% |
With phosphorus pentachloride; trichlorophosphate; at 0 - 105℃; for 16h; |
At 0C, 2,4-dihydroxypyrimidine-5-carboxylic acid (5.0g, 32.0mmol) was added to POCl3 (23mL) in batches, then PCl5 (23.3g) was added, and the mixture was moved to 105C and reacted for 16 hours, then concentrated , Add n-heptane (20 mL), filter, and concentrate the filtrate to obtain 6.5 g of product with a yield of 96%. |
| 92% |
With phosphorus pentachloride; trichlorophosphate; at 115℃; for 12h; |
To a solution of 2,4-dihydroxypyrimidine-5- carboxylic acid (5.0 g, 32.0 mmol) in POCI3 (50 mL) was added PCI5 (23.9 g, 115.2 mmol), and the resulting solution was heated to 115 C and stirred for 12 h. The solvent was evaporated and the crude residue was diluted with ethyl acetate (100 mL). The solid was filtered and the filtrate was concentrated to give the title compound as brown oil (6.2 g, 92%). |
| 78.8% |
With phosphorus pentachloride; trichlorophosphate; at 120℃; for 16.5h;Cooling with ice; |
Under ice-water bath stirring, phosphorus pentachloride (29.6g, 142mmol) was slowly added to 2,4-dihydroxypyrimidine-5-carboxylic acid (CAS: 23945-44-0, 6.2g, 40mmol) in batches. Phosphorus oxychloride (30ml) solution, stirred for 30min in an ice bath. Then the temperature was gradually increased to reflux (oil bath temperature 120C). After refluxing for 16 hours, it was cooled to room temperature. Carefully concentrate under reduced pressure, and the distillate is phosphorus oxychloride, which is quenched with warm water. After the residue was dissolved in dichloromethane, it was filtered. After the mother liquor was concentrated again, 6.7 g of oil was obtained, with a crude yield of 78.8%. Directly used in the next reaction |
| 70% |
With phosphorus pentachloride; trichlorophosphate; |
Method 31 2,4-Dichloro-5-chloroformylpyrimidine 5-Carboxy-2,4-dihydroxypyrimidine (19.0 g, 0.12 mol), phosphorus pentachloride (83.5 g, 0.40 mol); and phosphoryl chloride (28.3 ml, 0.30 mol) were heated at 114 C. for 5 hours. The resulting solution was cooled overnight and the volatiles removed by evaporation. The residue was purified by vacuum distillation to yield the title compound as a clear oil (17.85 g, 70%). M/z: 211. |
| 46% |
|
Example 16 2,4-DICHLOROPYRIMIDINE-5-CARBONYL CHLORIDE The title compound was prepared as described in the literature (Smith and Christensen, J. Org. Chem. 20:829, 1955) starting from 2,4-dihydroxypyrimidine-5-carboxylic acid. The compound was obtained by distillation; b.p. 90-100 C. (1.5 mm/Hg) in a yield of 46%; 1 H-NMR (CDCl3) δ 9.29. |
| 35% |
With phosphorus pentachloride; trichlorophosphate; |
REFERENTIAL EXAMPLE 26 2,4-Dichloro-5-chlorocarbonylpyrimidine STR43 Twenty grams of 2,4-dihydroxypyrimidine-5-carboxylic acid and 220 ml of phosphorus oxychloride were reacted under reflux for 10 hours. Thereafter, the reaction mixture was cooled to room temperature and 100 g of phosphorus pentachloride was added. The contents were again reacted at 120 C. for 7 hours. After completion of the reaction, phosphorus oxychloride was distilled off and the reaction product was purified by its distillation under reduced pressure (b.p. 130-140 C./1 mmHg, yield: 35%). |
|
With phosphorus pentachloride; trichlorophosphate; at 75℃;Heating / reflux; |
Prepared according to GB 1,182, 086: URACIL-5-CARBOXYLIC ACID MONOHYDRATE (15. 1 G, 86.7 mmol) was added in one portion to a suspension of phosphorus pentachloride (72.2 g, 346.7 mmol) in phosphorus oxychloride (50 mL). More phosphorus oxychloride (20 mL) was added. The reaction mixture was then heated up to 75 C and gradually to reflux overnight. The phosphorus oxychloride was then distilled off. The mixture was filtered, the precipitate washed with ethyl acetate and the filtrate was concentrated in vacuo to give the title compound (18.3 G) as a yellow oil 'H NMR (400 MHz, CDC13) : 9.26 (1H) ; 13C NMR (400 MHz, CDC13) : 126.44, 161.34, 162.19, 163.94, 164.43 |
|
With phosphorus pentachloride; trichlorophosphate; at 115℃; |
2,4-dichloro-5-pyrimidinecarbonyl chlorideA solution of 2,4-dihydroxy-pyrimidine-5-carboxylic acid (5Og) and phosphorous pentachloride (239g) in phosphorous oxychloride (230ml) was stirred at 1150C overnight. The excess phosphorous oxychloride was removed in vacuo and ethyl acetate (200ml) added to the residue. The mixture was filtered and the filtrate was concentrated to give yellow oil (78 g) as crude 2,4-dichloro-5-pyrimidinecarbonyl chloride which was used in the next step without further purification.1 H NMR (300MHz, D6-DMSO): δH 9.13(1 H, s). |
|
With phosphorus pentachloride; trichlorophosphate; for 16h;Heating / reflux; |
A suspension of 17.0 g (106 mmol) of 2,4-dihydroxypyrimidine-5-carboxylic acid hydrate and 81.1 g (370 mmol) of phosphorus pentachloride in 78 mL phosphorus oxycloride was heated at reflux for 16 h. The excess phosphorus oxycloride was removed by distillation and the residue was filtered. The filtered solids were rinsed with ethyl acetate and the filtrate was concentrated to give the title compound, which was used without further purification. 1H- NMR (CDC13) δ 9.25 (s, 1 H). |
|
With phosphorus pentachloride; trichlorophosphate; at 115℃; |
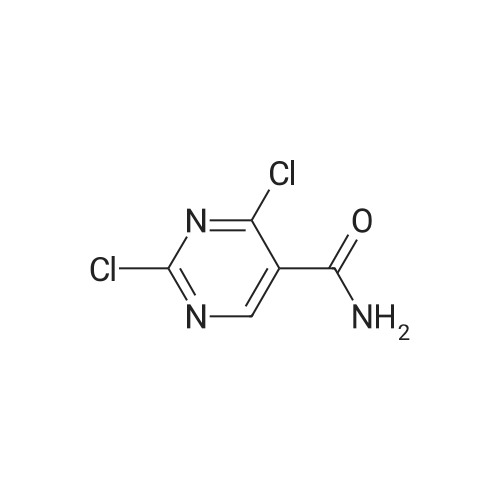
Example 17Synthesis of 5-Carboxyamide-2,4-DichloropyrimidineTo a 2 L round bottom flask equipped with water condenser and a CaCl2 drying tube, 2,4-dihydroxypyrimidine (25 g, 0.16 mole) was added to PCl5 (117 g, 0.56 mole), and POCl3 (250 ml, 2.6 mole). The mixture was heated at 115 C. overnight to give a clear, slightly light yellow solution. The mixture was cooled to room temperature, and was concentrated under reduced pressure to give pale yellowish oil.To this oil, anhydrous 1,4-dioxane (300 ml) was added and the mixture was cooled to 0 C. in an ice/water bath. 35 ml of NH3 in water (28%) was added dropwise to the mixture with stirring, temperature was kept below 5 C. The mixture changed from clear to white with precipitate forming, and was stirred for 1 hour at 0 C., reaction was followed by TLC (1:1 Hexanes:Ethyl Acetate). Ethyl acetate (700 ml) and water (500 ml) were added to the mixture, the 2 layers were separated. The organic layer was dried with Na2SO4, and filtered. The solution was concentrated under reduced pressure to give a light yellow solid. This light yellow solid was sonicated with methylene chloride (200 ml), and filtered to give a pale yellow solid (16 g). This pale yellow solid was dissolved into ethyl acetate (1.5 L) and washed with saturated NaHCO3 (500 ml). The organic layer was dried with Na2SO4, filtered, and concentrated under reduced pressure to give 13.1 g of product as a white solid (44% yield).1H NMR (DMSO-d6, 300 MHz): δ 8.86 (s, 1H), 8.14 (bs, 1H), 8.02 (bs, 1H). |
|
With phosphorus pentachloride; trichlorophosphate; at 115℃; |
Example 3 Synthesis of 5-carboxyamide-2,4-dichloropyrimidine To a 2 L round bottom flask equipped with water condenser and a CaCl2 drying tube, 2,4-dihydroxypyrimidine (25 g, 0.16 mole) was added to PCl5 (117 g, 0.56 mole), and POCl3 (250 ml, 2.6 mole). The mixture was heated at 115 C. overnight to give a clear, slightly light yellow solution. The mixture was cooled to room temperature, and was concentrated under reduced pressure to give pale yellowish oil. To this oil, anhydrous 1,4-dioxane (300 ml) was added and the mixture was cooled to 0 C. in an ice/water bath. 35 ml of NH3 in water (28%) was added dropwise to the mixture with stirring, temperature was kept below 5 C. The mixture changed from clear to white with precipitate forming, and was stirred for 1 hour at 0 C., reaction was followed by TLC (1:1 Hexanes:Ethyl Acetate). Ethyl acetate (700 ml) and water (500 ml) were added to the mixture, the 2 layers were separated. The organic layer was dried with Na2SO4, and filtered. The solution was concentrated under reduced pressure to give a light yellow solid. This light yellow solid was sonicated with methylene chloride (200 ml), and filtered to give a pale yellow solid (16 g). This pale yellow solid was dissolved into ethyl acetate (1.5 L) and washed with sat. NaHCO3 (500 ml). The organic layer was dried with Na2SO4, filtered, and concentrated under reduced pressure to give 13.1 g of product as a white solid (44% yield). 1H NMR (DMSO-d6, 300 MHz): δ 8.86 (s, 1H), 8.14 (bs, 1H), 8.02 (bs, 1H). |
| 4.38 g |
With phosphorus pentachloride; trichlorophosphate; for 4.5h;Reflux; |
5.1.32 2,4-Dichloropyrimidine-5-carboxamide (27) To a solution of 2,4-dihydroxypyrimidine-5-carboxylic acid (25) (2.98 g, 19.1 mmol) in POCl3 (7.10 mL, 76.4 mmol) was added PCl5 (13.1 g, 63.0 mmol) and the reaction mixture was refluxed for 4.5 h. After cooling, the reaction mixture was concentrated in vacuo. To the resulting residue, toluene (20 mL) was added and the mixture was concentrated in vacuo again. The residue was dissolved in CH2Cl2 (20 mL) and filtered. The filtrate was concentrated in vacuo to give 2,4-dichloropyrimidine-5-carbonyl chloride (26) as a yellow oil (4.38 g). This compound was used for next reaction without further purification. To a solution of 26 (4.38 g) in CH2Cl2 (5 mL) were added 28% NH3 aqueous solution (3 mL) and H2O (3 mL) at -10 C, and the reaction mixture was stirred at -10 C for 5 min. The organic solvent was removed under reduced pressure and the resulting precipitate was collected by filtration to give the title compound (3.13 g, 16.3 mmol, 85% from 25) as a pale yellow solid. 1H NMR (DMSO-d6) δ: 8.05 (1H, s), 8.17 (1H, s), 8.90 (1H, s); MS (ESI) m/z: 192 (M+H)+. |
|
With phosphorus pentachloride; trichlorophosphate; at 115℃; |
(a) (2,4-dichloropyrimidin-5-yl')(2-methylmorpholino')methanone (compound 3) Phosphorus pentachloride (128.1 g, 0.615 mol) was added portionwise to a stirred mixture of 2,4-dihydroxypyrimidine-5-carboxylic acid (compound 1) (26.7 g, 171 mmol, Alfa Aesar) in phosphorous oxychloride (122.2 ml_, 1.29 mol) at RT. The reaction mixture was heated to 115 C and stirred overnight. The reaction was cooled to RT and the volatiles were removed under vacuum. The residue was diluted with cyclohexane and filtered. The filtrate was evaporated under reduced pressure to give 2,4-dichloropyrimidine-5-carbonyl chloride (compound 2) as a yellow oil (37.9 g). This material was used in the next step without further purification. A solution of 2-methylmorpholine (Intermediate B) (4.91 g, 48.6 mmol, Enamine) and DIPEA (8.5 ml_, 48.8 mmol) in DCM (60 ml.) was added dropwise over 30 minutes to a stirred solution of 2,4-dichloropyrimidine-5-carbonyl chloride (compound 2) (9.34 g, 44.2 mmol) in DCM (242 ml.) at 0 C. The reaction mixture was stirred for 2 h at 0 C and then quenched with H20. The phases were separated and the organic phase washed with 0.5 M HCI, H20, dried over Na2S04 and then evaporated under reduced pressure. The residue was purified by flash column chromatography (Cyclohexane/EtOAc 6/4 to 4/6) to give desired (2,4- dichloropyrimidin-5-yl)(2-methylmorpholino)methanone (compound 3) as a colourless oil which solidified on standing (11.02 g, 90% yield). It will be appreciated by the skilled artisan that the above reaction may be carried out using 2-methylmorpholine hydrochloride salt (Intermediate B, hydrochloride salt), in which case an extra one equivalent of base (DIPEA) may be used in the reaction. Alternatively, 2-methylmorpholine hydrochloride salt may be converted to 2-methylmorpholine free base by treatment with a suitable base (e.g. DIPEA) prior to being employed in the reaction. MS (+ve ion electrospray): m/z 276 [MH+] |
|
With phosphorus pentachloride; at 80 - 105℃; |
The starting material Compound G was introduced in the reactor with POC13 and about 3 equivalents of PC15. In this case POC13 acted as solvent due to the very low solubility of Compound G in many organic solvents. The reaction temperature was increased from 80 C to 105 C in 3 hrs and then left at 105 C for additional 1-2 hrs. When the reaction completed, POC13 was distilled. Toluene was added to the residue and distilled in order to reduce the amount of POC13. Crude Compound F-i was distilled at around 7-8 mbar, with ajacketed temperature of about 130-13 5 C. Average yield 72% and average purity 94.9 % by GC-FID. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping