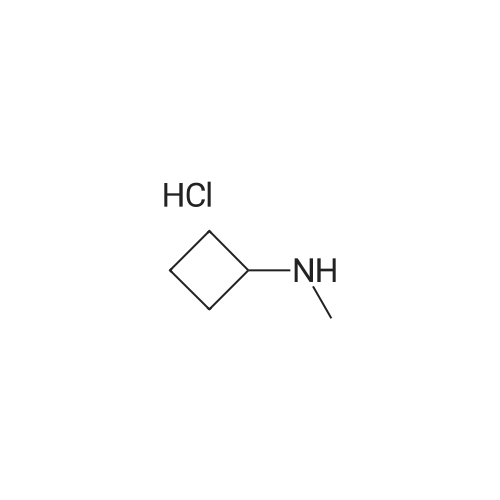
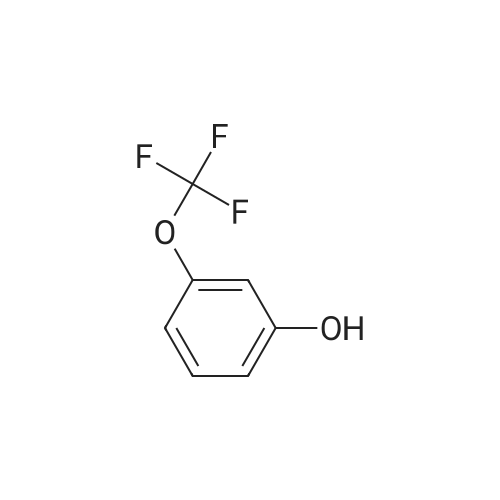
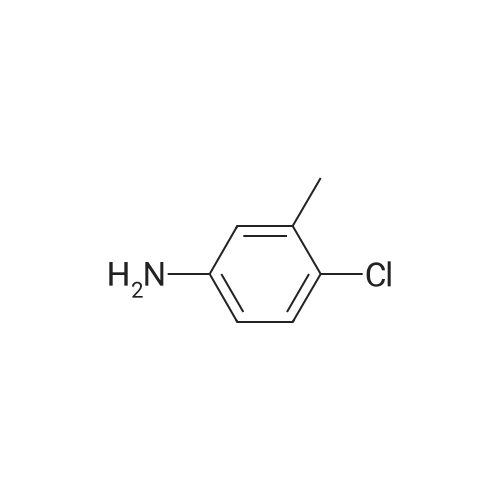
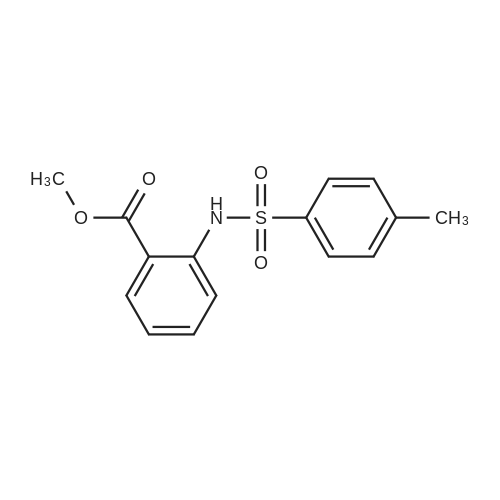
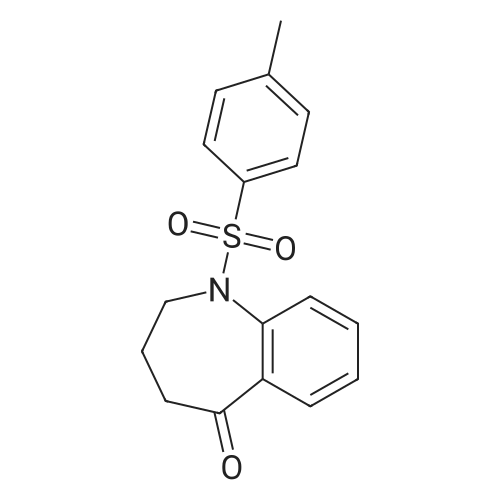
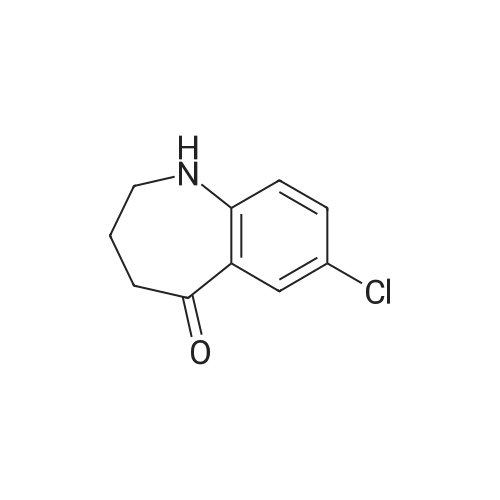
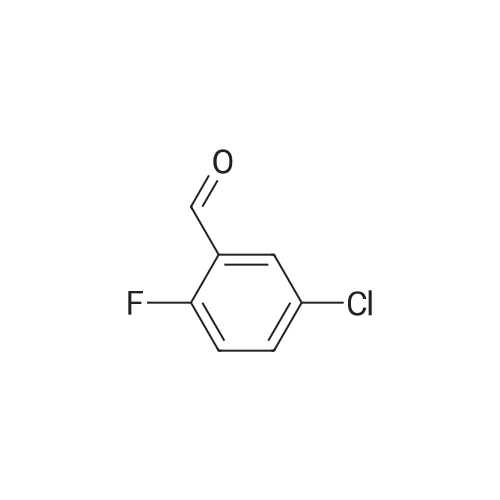
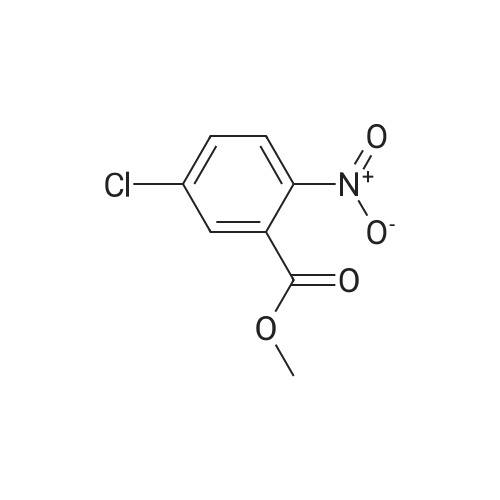
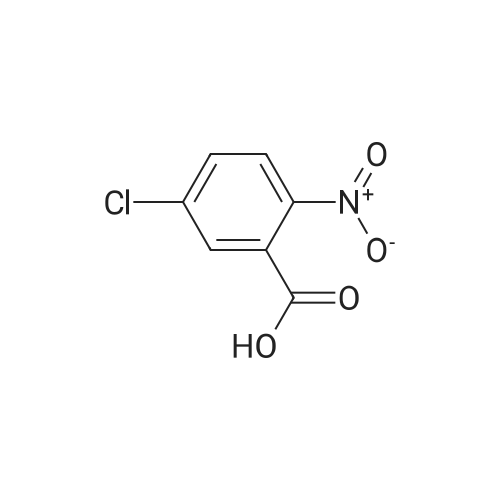
| 92% |
With potassium carbonate In acetonitrile for 8 h; Reflux |
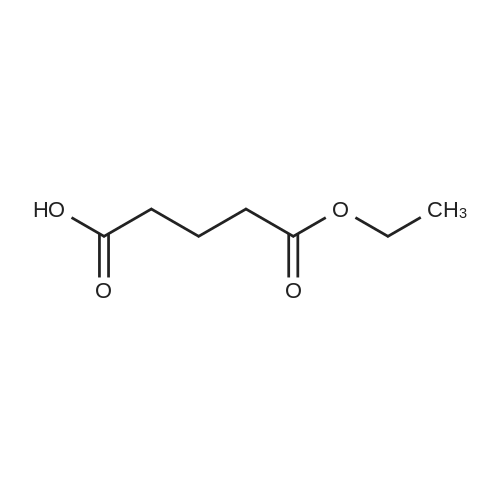
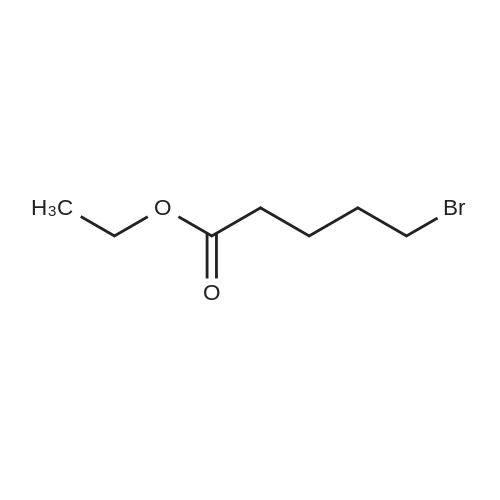
To a solution of 4-chlorophenol (2.0 g, 15.556 mmol, 1 equiv) in acetonitrile (60 mL) was added anhydrous potassium carbonate (4.3 g, 31 .1 13 mmol, 2 equiv) and ethyl 4-bromobutanoate (3.56 mL, 24.891 mmol, 1 .6 equiv). The reaction mixture was heated to reflux and stirred for 8 h. The progress of the reaction was monitored by TLC. After completion of reaction, the reaction mixture was allowed to cool to 27 °C, filtered the solid and washed with ethyl acetate (100 mL). The filtrate was concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography using 10percent ethyl acetate in hexane as eluent to obtain the title compound ethyl 4-(4-chlorophenoxy)butanoate (3.5 g, 92 percent yield) as colourless liquid. LCMS (ES) m/z = 242.9 [M+H]+. NMR (400 MHz, CDCI3): δ ppm 1 .25 (t, J = 6.8 Hz, 3 H), 2.06 - 2.06 (m, 2 H), 2.49 (t, J = 6.8 Hz, 2 H), 3.97 (t, J = 6.0 Hz, 2 H), 4.1 1 - 4.17 (m, 2 H), 6.80 (d, J = 8.4 Hz, 2 H), 7.21 (d, J = 8.8 Hz, 2 H). |
| 89% |
With potassium carbonate In N,N-dimethyl-formamide at 140℃; for 4 h; |
To a solution of 4-chlorophenol (10 g, 77.784 mmol, 1 equiv) in N,N- dimethylformamide (100 mL) was added anhydrous potassium carbonate (21 .5 g, 1 16.6 mmol, 2 equiv) and ethyl 4-bromobutanoate (16.7 mL, 1 16.677 mmol, 1 .5 equiv). The reaction mixture was heated to 140 °C and stirred for 4 h. The progress of the reaction was monitored by TLC. After completion of reaction, the reaction mixture was allowed to cool to 27 °C, filtered the solid and washed with ethyl acetate (700 mL). The filtrate was washed with water (2 x 200 mL), brine solution (100 mL), dried over anhydrous sodium sulphate, filtered and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography using 10 percent ethyl acetate in hexane as eluent to obtain ethyl 4-(4-chlorophenoxy)butanoate (17.0 g, 89 percent yield) as white solid. LCMS (ES) m/z = 243.1 [M+H]+. NMR (400 MHz, CDCI3): δ ppm 1 .25 (t, J = 7.2 Hz, 3 H), 2.06 - 2.12 (m, 2 H), 2.49 (t, J = 7.6 Hz, 2 H), 3.97 (t, J = 6.0 Hz, 2 H), 4.1 1 - 4.17 (m, 2 H), 6.80 (d, J = 8.8 Hz, 2 H), 7.21 (d, J = 8.8 Hz, 2 H). |
| 83% |
With potassium carbonate In acetoneReflux |
General procedure: General synthetic procedure for phenoxy acetic/butyric acid ethyl ester derivatives (3a-l) A mixture of substituted phenols 1a-f (0.05 mol) and 2a-b (0.075 mol) in dry acetone (40 ml) with anhydrous potassium carbonate (0.075 mol) were refluxed for 8-10 h. The reaction mixture was cooled and solvent removed by distillation. The residual mass was triturated with cold water to remove potassium carbonate, and extracted with ether (3 * 30 ml). The ether layer was washed with 10percent sodium hydroxide solution (3 * 30 ml) followed by water (3 * 30 ml) and then dried over anhydrous sodium sulfate and evaporated to afford compounds 3a-l. 4-(4-Chloro-phenoxy)-butyric acid ethyl ester (3b)
Yield 83percent; FT-IR (cm-1): 1738 (C=O), 1281 (C-O-C); 1H NMR (CDCl3): δ 1.35 (t, 3H, CH3 of ester), 2.26 (m, 2H, CH2), 2.75 (t, 2H, COCH2), 4.07 (t, 2H, OCH2), 4.31 (q, 2H, CH2 of ester), 6.88 (d, J = 8.80 Hz, 2H, Ar-H), 7.27 (d, J = 8.85 Hz, 2H, Ar-H); LC-MS m/z 243 (M + 1). Anal. Calcd. for C12H15ClO3: C, 59.39; H, 6.23. Found: C, 59.50; H, 6.16percent. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +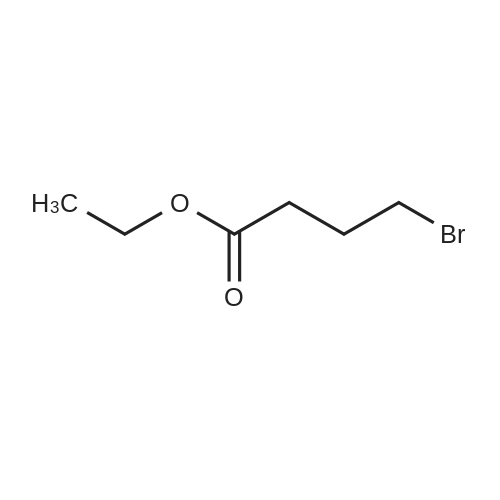

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping