| 92% |
With chloro-trimethyl-silane; for 15.0833h;Heating / reflux; |
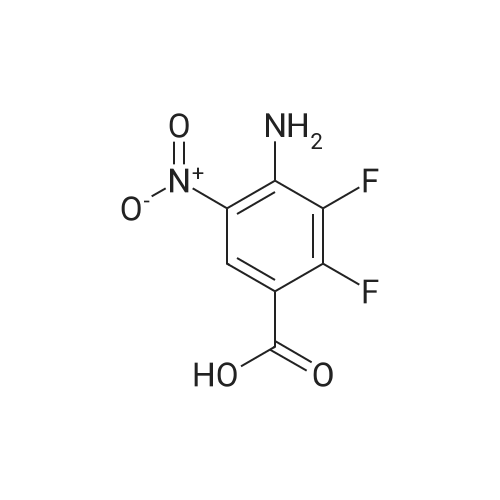
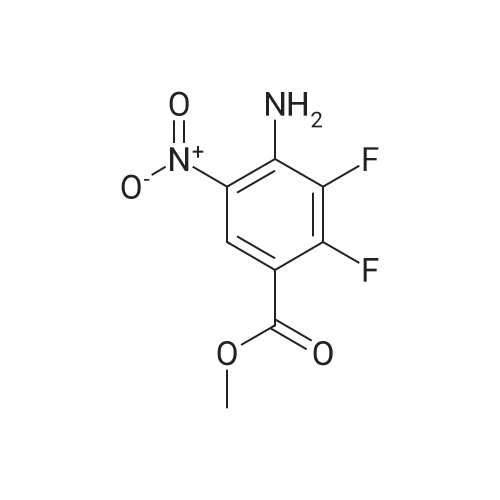
Step C; 4-Amino-2,3-difluoro-5-nitrobenzoic acid methyl ester; TMSCl (132 g, 1.21 mol, 2.0 equiv) was added over 5 minutes to a slurry of 4-amino-2,3- difluoro-5-nitrobenzoic acid (132.3 g, 0.607 mol, 1 equiv.) in 325 mL of MeOH. The mixture was heated at reflux for 15 hours. When the reaction was complete as determined by HPLC, the mixture was cooled in an ice-water bath for 45 minutes. The reaction mixture was then filtered and the cake was washed with 65 mL of MeOH. The wet cake was dried overnight at 55 0C under high vacuum to give 128.8 g (92%) of 4-amino-2,3-difluoro-5- nitrobenzoic acid methyl ester. HPLC was 97.9 a% (220 n?i) and 99.2 a % (254 nm). 1H NMR (400 MHz, DMSO-d6) delta 3.84 (3H, s, OMe), 8.1 (2H, br s, NH2), 8.43 (IH, apparent dd, J 1.9, 7.2, Ar-H). 19F NMR (376 MHz, D6 DMSO) delta -153.6, -129.2. 13C NMR (100 MHz, DMSO-d6) delta 52 (CH3O), 105 (C, d, J 10), 125 (CH, t, J 2.7,), 128 (CH, d, J 5), 140 (C-F, dd, J 244, 15,), 141 (C, dd, J 14, 5), 152 (C-F, dd, J263, 11), 162 (COO, t, J3). IR vmjcm l 3433, 3322, 1699, 1637, 1548, 1342, 1234. MS APCI (-) m/z 231 (M-I) detected. |
| 92% |
With chloro-trimethyl-silane; for 15h;Heating / reflux; |
TMSCl (132 g,1.21 mol, 2.0 equiv) was added over 5 minutes to a slurry of 4-amino-2,3-difluoro-5- nitrobenzoic acid (3) (132.3 g, 0.607 mol, 1 equiv) in 325 mL of MeOH. The mixture was heated at reflux for 15 hours. Once the reaction was complete by HPLC, the reaction mixture was cooled in an ice- water bath for 45 minutes. Then the reaction mixture was filtered and the cake was washed with 65 mL of MeOH. The wet cake was dried overnight at 55 C under high vacuum to provide 128.8 g (92%) of <strong>[284030-57-5]4-amino-2,3-difluoro-5-nitrobenzoic acid</strong> methyl ester (4). HPLC was 97.9 a% (220 nm) and 99.2 a % (254 nm). 1H NMR (400 MHz, d6 DMSO) delta 3.84 (3H, s, OMe)5 8.1 (2H, br s, NH2), 8.43 (IH, apparent dd, J 1.9, 7.2, Ar-H). 19F NMR (376 MHz, d6 DMSO) delta -153.6, -129.2. 13C NMR (100 MHz, d6 DMSO) delta 52 (CH3O), 105 (C, d, J 10), 125 (CH, t, J2.7,), 128 (CH5 d, J 5), 140 (C-F5 dd, J244, 15,), 141 EPO <DP n="66"/>(C, dd, J 14, 5), 152 (C-F, dd, J263, 11), 162 (COO, t, J 3). IR vmax/cm'' 3433, 3322, 1699, 1637, 1548, 1342, 1234. MS APCI (-) m/z 23.1 (M-I) detected.; TMSCl (132 g,1.21 mol, 2.0 equiv) was added over 5 minutes to a slurry of 4-amino-2,3-difluoro-5- nitrobenzoic acid (3) (132.3 g, 0.607 mol, 1 equiv) in 325 mL of MeOH. The mixture was EPO <DP n="78"/>heated at reflux for 15 hours. Once the reaction was complete by HPLC, the reaction mixture was cooled in an ice-water bath for 45 minutes. Then the reaction mixture was filtered and the cake was washed with 65 mL of MeOH. The wet cake was dried overnight at 55 C under high vacuum to provide 128.8 g (92%) of <strong>[284030-57-5]4-amino-2,3-difluoro-5-nitrobenzoic acid</strong> methyl ester (4). HPLC was 97.9 a% (220 nm) and 99.2 a % (254 nm). 1H NMR (400 MHz, d6 DMSO) delta 3.84 (3H, s, OMe), 8.1 (2H, br s, NH2), 8.43 (IH, apparent dd, J 1.9, 7.2, Ar-H). 19F NMR (376 MHz, d6 DMSO) delta -153.6, -129.2. 13C NMR (100 MHz, d6 DMSO) delta 52 (CH3O), 105 (C, d, J 10), 125 (CH, t, J2.7,), 128 (CH, d, J5), 140 (C-F, dd, J244, 15,), 141 (C, dd, J 14, 5), 152 (C-F, dd, J263, 11), 162 (COO, t, J 3). IR vmjcra l 3433, 3322, 1699, 1637, 1548, 1342, 1234. MS APCI (-) m/z 231 (M-I) detected. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping