Alternatived Products of [ 2754-32-7 ]
Product Details of [ 2754-32-7 ]
| CAS No. : | 2754-32-7 |
MDL No. : | MFCD00039516 |
| Formula : |
C10H18O2Si2
|
Boiling Point : |
No data available |
| Linear Structure Formula : | - |
InChI Key : | YBNBOGKRCOCJHH-UHFFFAOYSA-N |
| M.W : |
226.42
|
Pubchem ID : | 75989 |
| Synonyms : |
|
Application In Synthesis of [ 2754-32-7 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 2754-32-7 ]
- 1
-
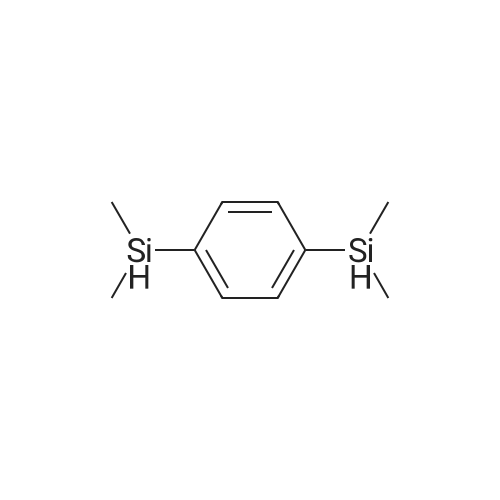 [ 2488-01-9 ]
[ 2488-01-9 ]

-
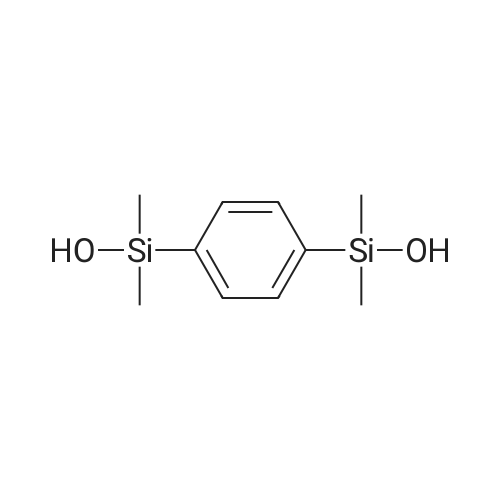 [ 2754-32-7 ]
[ 2754-32-7 ]
| Yield | Reaction Conditions | Operation in experiment |
| 92% |
|
A double-necked round-bottom flask was set up with a reaction device on which a reflux tube was placed, and the inside was dried and removed, and replaced with a nitrogen atmosphere. Measure the pre-dried anhydrous methanol (250 ml) into a reaction device and place the reaction device in an ice water bath. Under a nitrogen or argon atmosphere, 6.3 g (274.2 mmol) of sodium metal cut into small pieces was carefully added successively. After the metal sodium had completely reacted with methanol and no hydrogen bubbles were generated, a first solution of sodium methoxide was prepared. Weighing 17.8 g (91.4 millimoles) of dioxane of structural formula (A-1) in a feeding tube, Pre-dried anhydrous methanol (50 ml) was added to the feed tube to prepare a bis-decane solution of structural formula (A-1), which was the second solution. The rate of dropwise addition was controlled and the second solution was slowly fed into the first solution in the reaction apparatus. Hydrogen bubbles continued to be generated during the addition. After the dropwise addition was completed, stirring was continued at room temperature for 10 minutes until the generation of hydrogen gas was moderated until it subsided, which was the third solution. Then, a mixed solution of 11.0 g (274.2 millimoles) and water (150 ml) of pre-formulated sodium hydroxide was slowly added dropwise to the third solution, and stirring was continued at room temperature for 20 minutes. This is the fourth solution, and the hydrolysis reaction has been completed so far. After the reaction was completed, a saturated aqueous solution of ammonium chloride was gradually added to the fourth solution for neutralization, and the mixture was cooled in an ice-water bath and stirred for 20 minutes. This was the fifth solution. The fifth solution was transferred to a separatory funnel and extracted with ether (200 ml/3 times). The organic layer was collected and washed with saturated brine (300 ml), dried over anhydrous magnesium sulfate and filtered. The filtrate was concentrated under reduced pressure to give the crude product of the white solid as the phenylephrine of formula (I-1). N-hexane was added to the crude white solid product of the phenylenedioxylanol of formula (I-1). After thorough and uniform mixing, the mixture was collected by suction filtration. The white filter cake was washed with n-hexane, and the white solid product was collected and dried to obtain 19.1 g of the bis-stanol product of formula (I-1). The yield was 92%. |
| 92% |
|
Synthesis of compound (I-1): Setup a reaction device, such as a reflux pipe, on a two-necked round bottom flask. Dry the inside of the two-necked round bottom flask. Introduce nitrogen gas into the two-necked round bottom flask. Put 250 mL of pre-dried anhydrous methanol in the reaction device. Place the reaction device in an ice water bath. Chips of 6.3 g (274.2 mmol) of metallic sodium are put in the reaction device immersed in the ice water bath under nitrogen gas or argon gas atmosphere one by one and carefully. After the metallic sodium has reacted with methanol completely and no hydrogen bubbles have been generated, the first solution of sodium methoxide is produced. Then, 17.8 g (91.4 mmol) of disilane with structural formula (A-1) and 50 mL of pre-dried anhydrous methanol are introduced into the feeding pipe to form a disilane (expressed by structural formula (A-1)) solution known as the second solution. Drip the second solution slowly to the first solution in the reaction device while hydrogen bubbles are being continuously generated. Afterward, the mixture of first and second solutions are stirred for 10 minutes at room temperature while the generation of hydrogen gas is fading out, thereby producing the third solution. Then, an aqueous solution of a mixture of 11.0 g (274.2 mmol) of sodium hydroxide and 150 mL of water is dripped slowly to the third solution while the third solution is being stirred for 20 minutes, so as to produce the fourth solution. At this point in time, the hydrolysis process is finished, and a saturated ammonium chloride aqueous solution is added to the fourth solution to trigger neutralization therebetween while the fourth solution is being cooled and stirred in an ice water bath for 20 minutes to produce the fifth solution. Transfer the fifth solution to a separatory funnel to undergo extraction with 200 mL of ether thrice, and then collect the organic supernatant before rinsing it with 300 mL of saturated brine solution for the sake of drying. A drying process is performed with anhydrous magnesium sulfate, and then filtration is performed so that the filtrate is depressurized and concentrated to obtain a crude white solid product of phenylene disilanol with structural formula (I-1). Add n-hexane to the crude white solid product of phenylene disilanol with structural formula (I-1) and mix them. Collect the solid precipitate by suction filtration. Rinse the white solid with n-hexane. Collect the white solid product and dry it to obtain 19.1 g of disilanol with structural formula (I-1) at a yield of 92%. Referring to FIG. 4, it shows the 1H-NMR spectrum for phenylene disilanol according to an embodiment of the present invention. |
Reference:
[1]Chemical Communications,2016,vol. 52,p. 10625 - 10628
[2]Angewandte Chemie - International Edition,2019,vol. 58,p. 6380 - 6384
Angew. Chem.,2019,vol. 131,p. 6446 - 6450,5
[3]Angewandte Chemie - International Edition,2011,vol. 50,p. 7533 - 7536
[4]Patent: TWI606055,2017,B .Location in patent: Paragraph 0035
[5]Patent: US2018/201631,2018,A1 .Location in patent: Paragraph 0042
[6]New Journal of Chemistry,2002,vol. 26,p. 1536 - 1538
[7]Tetrahedron Letters,1994,vol. 35,p. 6329 - 6330
[8]Angewandte Chemie - International Edition,2008,vol. 47,p. 7938 - 7940
[9]Chemical Communications,2009,p. 5302 - 5304
[10]European Journal of Inorganic Chemistry,2010,p. 5675 - 5684
[11]Chemical Communications,2021,vol. 57,p. 3660 - 3663
- 2
-
 [ 2488-01-9 ]
[ 2488-01-9 ]

-
 [ 2754-32-7 ]
[ 2754-32-7 ]

-
C90H146O8Si18
[ No CAS ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping






