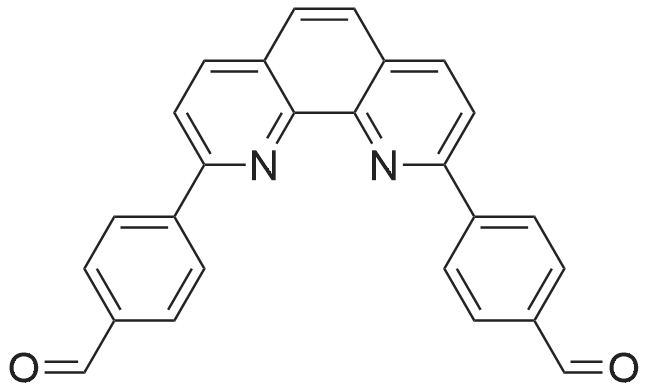
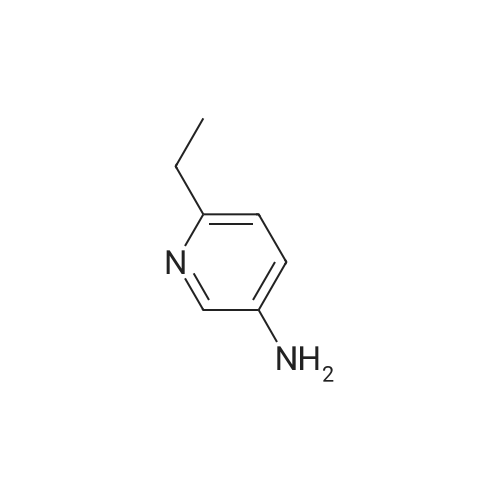
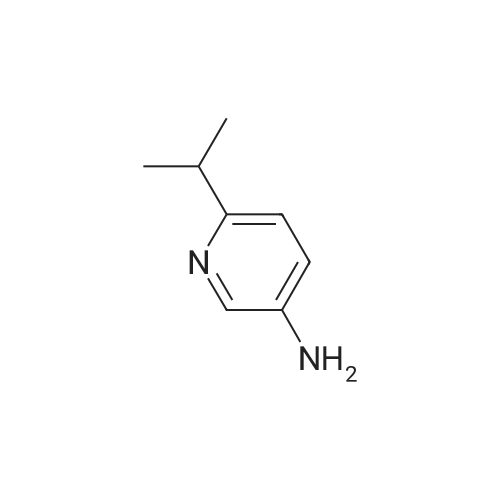
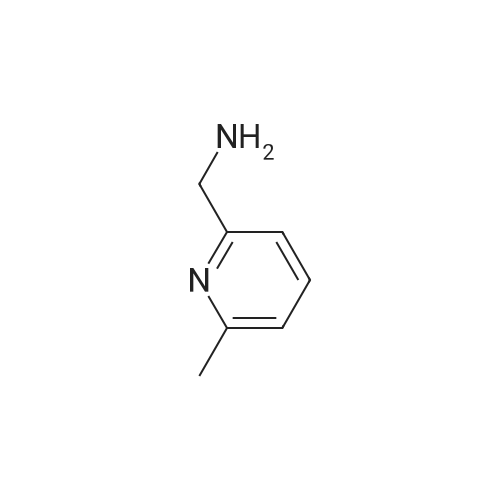
|
With potassium carbonate; In dimethyl sulfoxide; at 115℃; for 1.5h;microwave irradiation; |
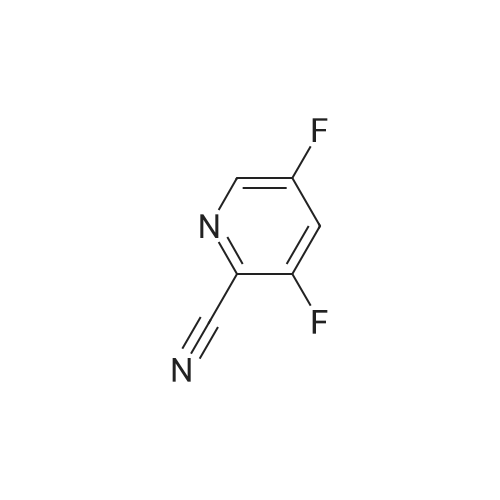
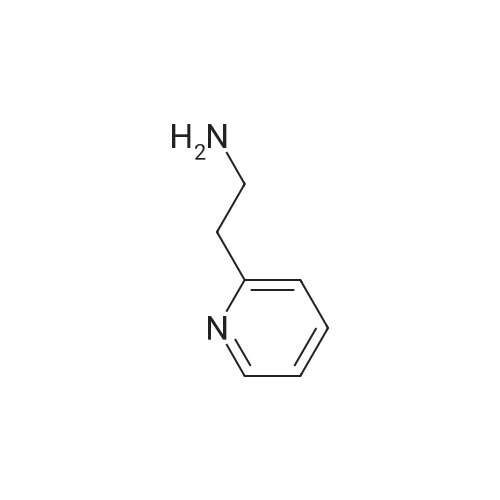
Example 133Synthesis of 3-[(2-pyridin-2-yl)ethylamino)-5-[4-(quinolin-3-yl)-9H-carbazol-9-yl]pyridine-2-carboxamideStage 1:841 mg of <strong>[298709-29-2]2-cyano-3,5-difluoropyridine</strong>, 880 mg of 2-(2-aminoethyl)pyridine and 1.658 g of potassium carbonate in 12.5 ml of dimethyl sulphoxide are introduced into a 20 ml microwave tube reactor.The mixture is then heated in a microwave for 1 and a half hours at 115° C.The reaction medium is run into 100 ml of water and 100 ml of ethyl acetate.The aqueous phase is re-extracted twice with 50 ml of ethyl acetate.The combined organic phases are washed with water and then with a saturated aqueous solution of sodium chloride, dried over sodium sulphate and concentrated under reduced pressure.After purification by flash chromatography on 70 g of silica gel, elution being carried out with a mixture of ethyl acetate and cyclohexane (40/60 by volume), and the first eluted product being recovered, 549 mg of 2-cyano-5-fluoro-3-(2-pyridin-2-ylethylamino)pyridine are thus obtained in the form of a beige powder, the characteristics of which are the following:1H NMR spectrum (400 MHz, DMSO-d6, delta ppm): 3.02 (t, J=7.1 Hz, 2 H) 3.57 (q, J=6.8 Hz, 2 H) 6.94 (broad s, 1 H) 7.15-7.27 (m, 2 H) 7.33 (d, J=7.8 Hz, 1 H) 7.71 (td, J=7.6, 1.8 Hz, 1 H) 7.86 (d, J=2.4 Hz, 1 H) 8.51 (d, J=4.9 Hz, 1 H). |
|
With potassium carbonate; In dimethyl sulfoxide; at 115℃; for 1.5h;Inert atmosphere; Microwave irradiation; |
841 mg of <strong>[298709-29-2]2-cyano-3,5-difluoropyridine</strong>, 880 mg of 2-(2-aminoethyl)pyridine and 1.658 g of potassium carbonate in 12.5 ml of dimethyl sulphoxide are charged to a 20 ml microwave tube-reactor.The mixture is then microwave-heated for 1.5 hours at 115° C.The reaction medium is run into 100 ml of water and 100 ml of ethyl acetate.The aqueous phase is re-extracted twice with 50 ml of ethyl acetate.The combined organic phases are washed with water and then with a saturated aqueous solution of sodium chloride, dried over sodium sulphate and concentrated under reduced pressure.After flash chromatography on silica gel (40-63 mum), elution being carried out with a mixture of ethyl acetate and cyclohexane (40:60 v/v), with the first eluted product being collected, 549 mg of 2-cyano-5-fluoro-3-(2-pyridin-2-ylethylamino)pyridine are obtained in the form of a beige powder, the characteristics of which are the following:1H NMR spectrum (400 MHz, delta in ppm, DMSO-d6): 3.02 (t, J=7.1 Hz, 2H); 3.57 (q, J=6.8 Hz, 2H); 6.94 (broad s, 1H); 7.15 to 7.27 (m, 2H); 7.33 (d, J=7.8 Hz, 1H); 7.71 (td, J=7.6 and 1.8 Hz, 1H); 7.86 (d, J=2.4 Hz, 1H); 8.51 (d, J=4.9 Hz, 1H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping