|
|
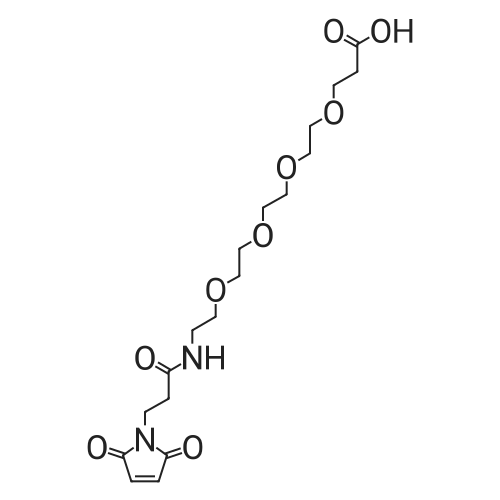
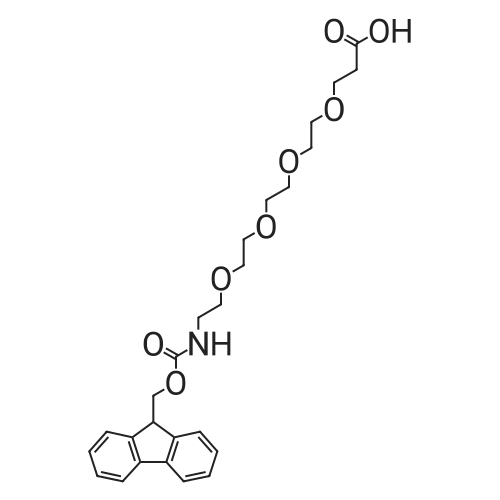
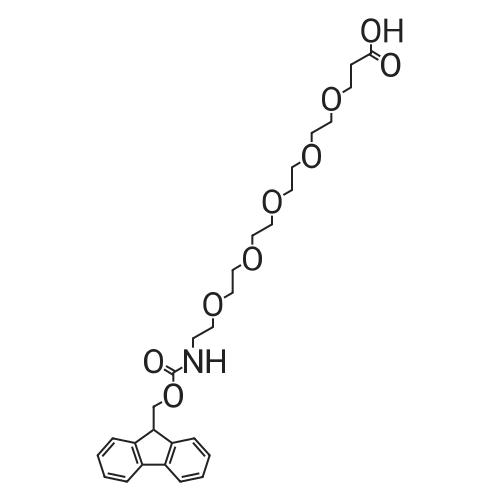
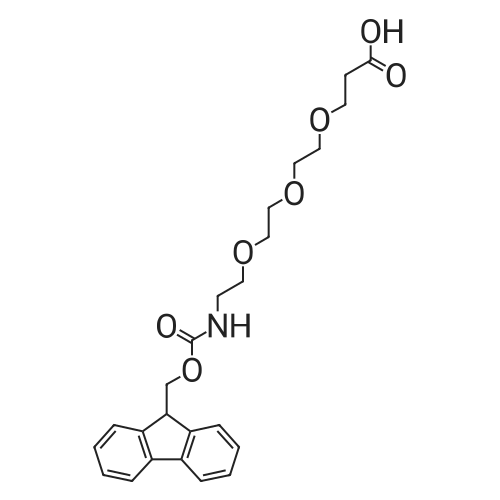
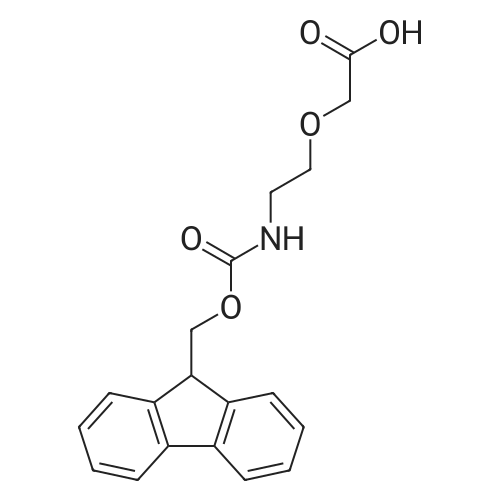
The title compound was synthesized using solid phase peptide synthesis as described herein. 2-(2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)ethoxy)acetic acid (1543 mg) was dissolved in 10 mL dioxane, and the solvent was concentrated under reduced pressure. (The procedure was repeated twice). The material was lyophilized overnight. The dioxane-dried amino acid was dissolved in 20 mL sieve-dried dichloromethane to which was added N,N- diisopropylethylamine (4.07 mL). The solution was added to a 2-chlorotrityl solid support resin (8000 mg), which was previously washed (twice) with sieve-dried dichloromethane. The mixture of resin and amino acid was shaken at ambient temperature for 4 hours, drained, washed with 17:2: 1 dichloromethane:methanol:N,N-diisopropylethylamine, and washed three times with N,N- dimethylformamide. The mixture was then washed three more times, alternating between sieve-dried dichloromethane and methanol. The loaded resin was dried in a vacuum oven at 40 C. The resin loading was determined by quantitative Fmoc-loading test measuring absorbance at 301 nm of a solution obtained by deprotecting a known amount of resin by treatment with 20% piperidine in N,N- dimethylformamide. All Fmoc deprotection steps were performed by treatment of the resin with 20% piperidine in N,N-dimethylformamide for 20 minutes followed by a washing step with N,N- dimethylformamide. Coupling of the amino acids (R)-2-((((9H-fluoren-9- yl)methoxy)carbonyl)amino)-3-sulfopropanoic acid and subsequently l-(2,5-dioxo-2,5-dihydro-lH- pyrrol-l-yl)-3 -oxo-7, 10, 13, 16-tetraoxa-4-azanonadecan-19-oic acid was done by activation of 4 equivalents of amino acid with 4 equivalents of ((lH-benzo[d][l,2,3]triazol-l-yl)oxy)tri(pyrrolidin-l- yl)phosphonium hexafluorophosphate(V) and 8 equivalents of N,N-diidopropylethylamine in N,N- dimethylformamide for one minute followed by incubation with the resin for one hour. The title compound was cleaved from the resin by treatment with 5 % trifluoroacetic acid in dichloromethane for 30 minutes. The resin was filtered, and the filtrate was concentrated under reduced pressure to yield the title compound which was used in the next step without further purification. MS (ESI) m/e 669.0 (M+H)+ |
|
|
The title compound was synthesized using solid phase peptide synthesis as described herein. 2-(2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)ethoxy)acetic acid (1543 mg) was dissolved in 10 mLdioxane, and the solvent was concentrated under reduced pressure. (The procedure was repeated twice). The material was lyophilized overnight. The dioxane-dried amino acid was dissolved in 20 mL sieve-dried dichloromethane to which was added N,N-diisopropylethylamine (4.07 mL). The solution was added to a 2-chlorotrityl solid support resin (8000 mg), which was previously washed(twice) with sieve-dried dichloromethane. The mixture of resin and amino acid was shaken at ambient temperature for 4 hours, drained, washed with 17:2:1 dichloromethane:methanol:N,Ndiisopropylethylamine, and washed three times with N,N-dimethylformamide. The mixture was then washed three more times, alternating between sieve-dried dichloromethane and methanol. The loaded resin was dried in a vacuum oven at 40 C. The resin loading was determined by quantitative Fmoc20 loading test measuring absorbance at 301 nm of a solution obtained by deprotecting a known amountof resin by treatment with 20% piperidine in N,N-dimethylformamide. All Fmoc deprotection steps were performed by treatment of the resin with 20% piperidine in N,N-dimethylformamide for 20 minutes followed by a washing step with N,N-dimethylformamide. Coupling of the amino acids (R)2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-sulfopropanoic acid and subsequently 1 -(2,5-dioxo-2,5-dihydro- 1 H-pyrrol- 1 -yl)-3 -oxo-7, 10,13,1 6-tetraoxa-4-azanonadecan- 19-oic acid was done by activation of 4 equivalents of amino acid with 4 equivalents of ((1H-benzo[d][1,2,3]triazol-1- yl)oxy)tri(pyrrolidin- 1 -yl)phosphonium hexafluorophosphate(V) and 8 equivalents of N,Ndiidopropylethylamine in N,N-dimethylformamide for one minute followed by incubation with the resin for one hour. The title compound was cleaved from the resin by treatment with 5 %trifluoroacetic acid in dichloromethane for 30 minutes. The resin was filtered, and the filtrate was concentrated under reduced pressure to yield the title compound which was used in the next step without further purification. MS (ESI) m/e 669.0 (M+H). |
|
|
The title compound was synthesized using solid phase peptide synthesis as described herein. 2-(2-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)ethoxy)acetic acid (1543 mg) was dissolved in 10 mL dioxane, and the solvent was concentrated under reduced pressure. (The procedure was repeated twice). The material was lyophilized overnight. The dioxane-dried amino acid was dissolved in 20 mL sieve-dried dichloromethane to which was added N,N-diisopropylethylamine (4.07 mL). The solution was added to a 2-chlorotrityl solid support resin (8000 mg), which was previously washed (twice) with sieve-dried dichloromethane. The mixture of resin and amino acid was shaken at ambient temperature for 4 hours, drained, washed with 17:2:1 dichloromethane:methanol:N,N-diisopropylethylamine, and washed three times with N,N-dimethylformamide. The mixture was then washed three more times, alternating between sieve-dried dichloromethane and methanol. The loaded resin was dried in a vacuum oven at 40 C. The resin loading was determined by quantitative Fmoc-loading test measuring absorbance at 301 nm of a solution obtained by deprotecting a known amount of resin by treatment with 20% piperidine in N,N-dimethylformamide. All Fmoc deprotection steps were performed by treatment of the resin with 20% piperidine in N,N-dimethylformamide for 20 minutes followed by a washing step with N,N-dimethylformamide. Coupling of the amino acids (R)-2-((((9H-fluoren-9-yl)methoxy)carbonyl)amino)-3-sulfopropanoic acid and subsequently <strong>[1263045-16-4]1-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)-3-oxo-7,10,13,16-tetraoxa-4-azanonadecan-19-oic acid</strong> was done by activation of 4 equivalents of amino acid with 4 equivalents of ((1H-benzo[d][1,2,3]triazol-1-yl)oxy)tri(pyrrolidin-1-yl)phosphonium hexafluorophosphate(V) and 8 equivalents of N,N-diidopropylethylamine in N,N-dimethylformamide for one minute followed by incubation with the resin for one hour. The title compound was cleaved from the resin by treatment with 5% trifluoroacetic acid in dichloromethane for 30 minutes. The resin was filtered, and the filtrate was concentrated under reduced pressure to yield the title compound which was used in the next step without further purification. MS (ESI) m/e 669.0 (M+H)+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping