|
With dimethyl sulfoxide; In ethyl acetate; |
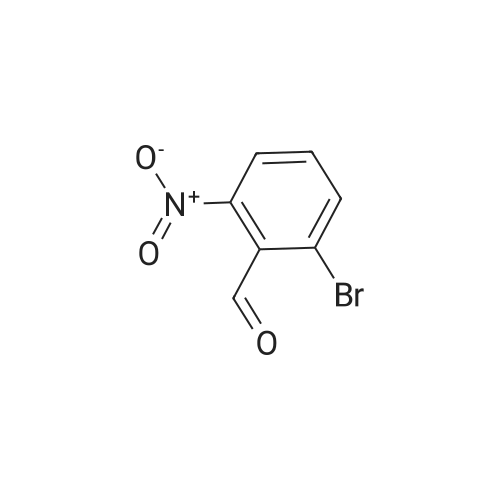
Preparation Example 1 2-Amino-9-bromoquinoline After stirring <strong>[20357-21-5]2-bromo-6-nitrobenzaldehyde</strong> (30.4 g), magnesium oxide (75 g) and dimethyl sulfoxide (11.3 ml) sufficiently for 1 minute, diethyl (cyanomethyl)phosphonate (25.8 ml) was added thereto and the mixture was stirred for further 2 hours. After the completion of stirring, the mixture was left stand overnight. Then, ethyl acetate was added thereto, and the mixture was stirred and then filtered. The filtrate was concentrated and the residue was purified by silica gel column chromatography (ethyl acetate), to give 32 g of 3-(2-bromo-6-nitrophenyl)-2-propenenitrile (E-isomer:Z-isomer=3:1). 1H-NMR (CDCl3) delta (ppm): 5.63 (d, J=16.5 Hz, E-isomer 1H), 5.81 (d, J=10.8 Hz, Z-isomer 1H), 7.42-7.52 (m, E-isomer 1H, Z-isomer 2H), 7.56 (d, J=16.5 Hz, E-isomer 1H), 7.90-8.16 (m, E-isomer 2H, Z-isomer 2H). |
|
With dimethyl sulfoxide; In ethyl acetate; |
Production Example 1b 2-Amino-5-bromoquinoline <strong>[20357-21-5]2-Bromo-6-nitrobenzaldehyde</strong> (30.4 g), magnesium oxide (75 g) and dimethyl sulfoxide (11.3 ml) were sufficiently stirred for one minute. Then, to the mixture was added diethyl (cyanomethyl) phosphonate (25.8 ml) and the mixture was stirred for further 2 hours. The stirring was stopped and the reaction mixture was allowed to stand overnight. Thereafter, ethyl acetate was added thereto and the resulting mixture was stirred, followed by filtering. The filtrate was concentrated and the residue was purified by silica gel column chromatography (ethyl acetate), to give 32 g of 3-(2-bromo-6-nitrophenyl)-2-propenenitrile (E isomer:Z isomer=3:1). 1H-NMR(CDCl3) delta (ppm): 5.63 (d,J=16.5Hz,E-isomer1H), 5.81(d,J=10.8Hz,Z-isomer 1H), 7.42-7.52(m,E-isomer 1H,Z-isomer 2H), 7.56(d,J=16.5Hz,E-isomer 1H), 7.90-8.16(m,E-isomer 2H, Z-isomer 2H). |
|
With dimethyl sulfoxide; In ethyl acetate; |
PRODUCTION EXAMPLE 1b 2-Amino-5-bromoquinoline <strong>[20357-21-5]2-Bromo-6-nitrobenzaldehyde</strong> (30.4 g), magnesium oxide (75 g) and dimethyl sulfoxide (11.3 ml) were sufficiently stirred for one minute. Then, to the mixture was added diethyl (cyanomethyl)phosphonate (25.8 ml) and the mixture was stirred for further 2 hours. The stirring was stopped and the reaction mixture was allowed to stand overnight. Thereafter, ethyl acetate was added thereto and the resulting mixture was stirred, followed by filtering. The filtrate was concentrated and the residue was purified by silica gel column chromatography (ethyl acetate), to give 32 g of 3-(2-bromo-6-nitrophenyl)-2-propenenitrile (E isomer:Z isomer=3:1). 1H-NMR(CDCl3) delta (ppm): 5.63(d, J=16.5 Hz, E-isomer1H), 5.81(d, J=10.8 Hz, Z-isomer 1H), 7.42-7.52(m, E-isomer 1H,Z-isomer 2H), 7.56(d, J=16.5 Hz, E-isomer 1H), 7.90-8.16(m, E-isomer 2H, Z-isomer 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +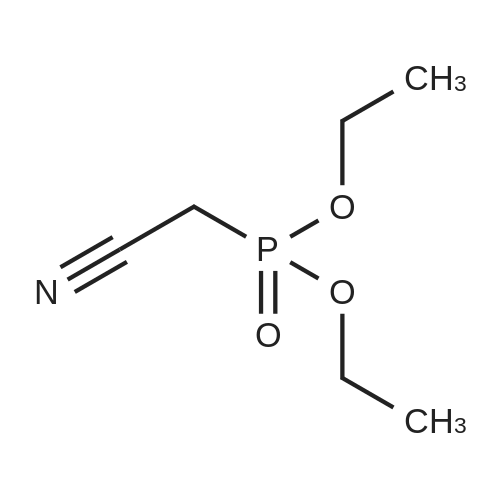

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping