|
|
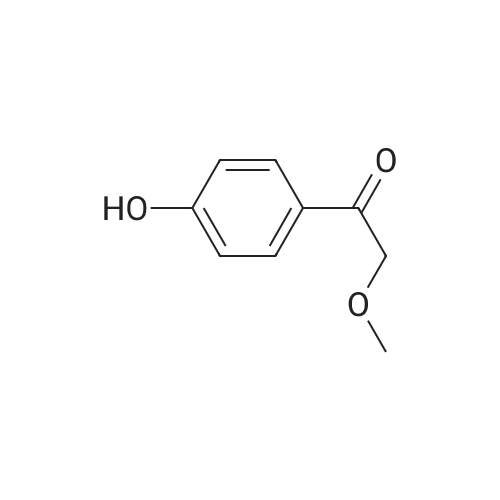
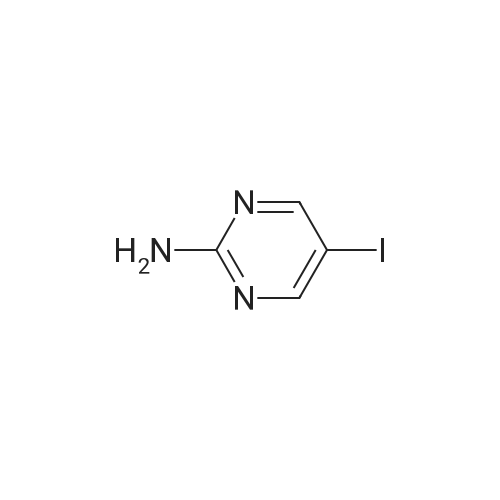
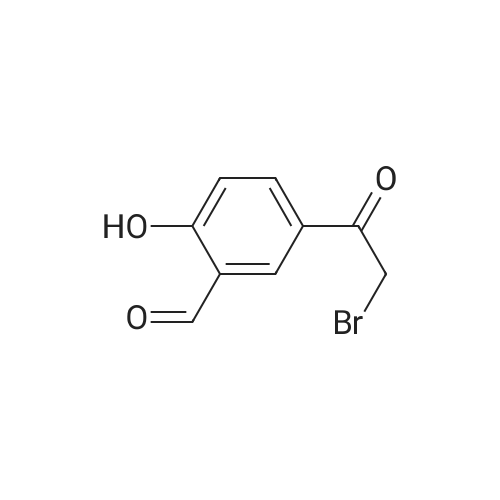
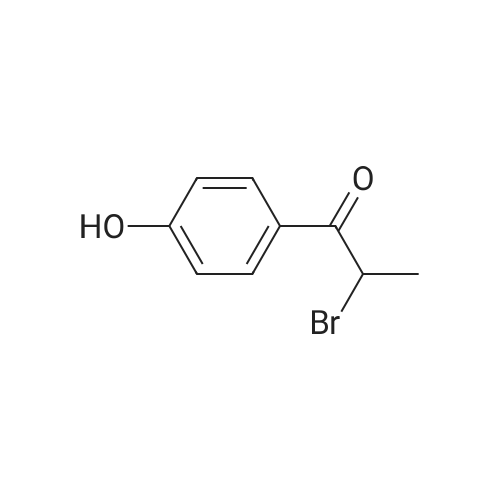
646 mg (corresponding to 3.0 mmol) of 2-bromo-4'-hydroxyacetophenone and 668 mg (corresponding to 3.0 mmol) of <strong>[1445-39-2]2-amino-5-iodopyrimidine</strong> were dissolved in 20 mL of acetonitrile. The resulting solution was refluxed in an oil bath at 110C for 8 hours. After the completion of the reaction, the reaction solution was cooled down to room temperature, and precipitates were filtered and recovered. The precipitates were washed with acetonitrile and dried under reduced pressure. The resulting crude crystals were suspended in a mixed solution of 10 mL of water and 10 mL of methanol. Then, about 15 mL of a saturated sodium hydrogencarbonate solution was added thereto, and the mixture was sonicated for 3 minutes using an ultrasonic washing machine. Precipitates were filtered and recovered from the resulting mixture, sufficiently washed with water, and dried under reduced pressure, to obtain 737 mg (corresponding to 2.19 mmol) of 2-(4'-hydroxyphenyl)-6-iodoimidazo[1,2-a]pyrimidine (Fig. 1-8, Step 2). |
|
|
646 mg (corresponding to 3.0 mmol) of 2-bromo-4'-hydroxyacetophenone and 668 mg (corresponding to 3.0 mmol) of <strong>[1445-39-2]2-amino-5-iodopyrimidine</strong> were dissolved in 20 mL of acetonitrile. The resulting solution was refluxed in an oil bath at 110C for 8 hours. After the completion of the reaction, the reaction solution was cooled down to room temperature, and precipitates were filtered and recovered. The precipitates were washed with acetonitrile and dried under reduced pressure. The resulting crude crystals were suspended in a mixed solution of 10 mL of water and 10 mL of methanol. Then, about 15 mL of a saturated sodium hydrogencarbonate solution was added thereto, and the mixture was sonicated for 3 minutes using an ultrasonic washing machine. Precipitates were filtered and recovered from the resulting mixture, sufficiently washed with water, and dried under reduced pressure, to obtain 737 mg (corresponding to 2.19 mmol) of 2-(4'-hydroxyphenyl)-6-iodoimidazo[1,2-a]pyrimidine (Fig. 4, Step 2). The NMR measurement results of the resulting 2-(4'-hydroxyphanyl)-6-iodoimidazo[1,2-a]pyrimidine (internal standard: dimethylformamide) are shown below. NMR apparatus employed: JNM-ECP-500 (manufactured by Japan Electron Optics Laboratory Co., Ltd. (JEOL)) 1H-NMR (solvent: dimethylformamide-d7, resonance frequency: 500 MHz): delta 9.80 (br. s, 1H), 9.35 (d, J = 2.3 Hz, 1H), 8.60 (d, J = 2.3 Hz, 1H), 8.23 (s, 1H), 7.94-7.90 (m, 2H), 6.98-6.94 (m, 2H). 13C-NMR (solvent: dimethylformamide-d7, resonance frequency: 125 MHz): delta 158.87, 154.00, 147.18, 146.77, 139.07, 127.68, 124.50, 115.85, 106.10, 73.46. |
|
With sodium hydrogencarbonate; In methanol; water; acetonitrile; |
, Step 1). 646 mg (corresponding to 3.0 mmol) of 2-bromo-4'-hydroxyacetophenone and 668 mg (corresponding to 3.0 mmol) of <strong>[1445-39-2]2-amino-5-iodopyrimidine</strong> were dissolved in 20 mL of acetonitrile. The resulting solution was refluxed in an oil bath at 110C for 8 hours. After the completion of the reaction, the reaction solution was cooled down to room temperature, and precipitates were filtered and recovered. The precipitates were washed with acetonitrile and dried under reduced pressure. The resulting crude crystals were suspended in a mixed solution of 10 mL of water and 10 mL of methanol. Then, about 15 mL of a saturated sodium hydrogencarbonate solution was added thereto, and the mixture was sonicated for 3 minutes using an ultrasonic washing machine. Precipitates were filtered and recovered from the resulting mixture, sufficiently washed with water, and dried under reduced pressure, to obtain 737 mg (corresponding to 2.19 mmol) of 2-(4'-hydroxyphenyl)-6-iodoimidazo[1,2-a]pyrimidine ( |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping