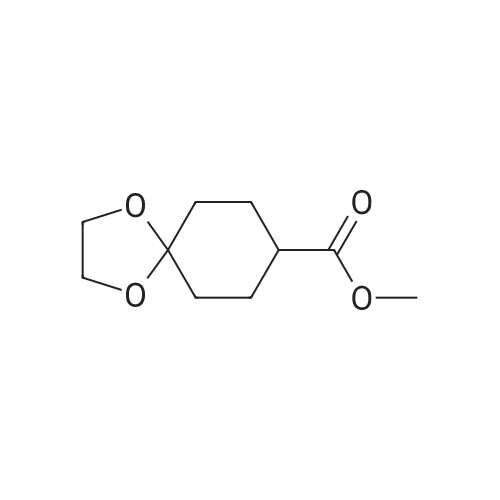
| 67% |
With toluene-4-sulfonic acid; In water; acetone;Reflux; |
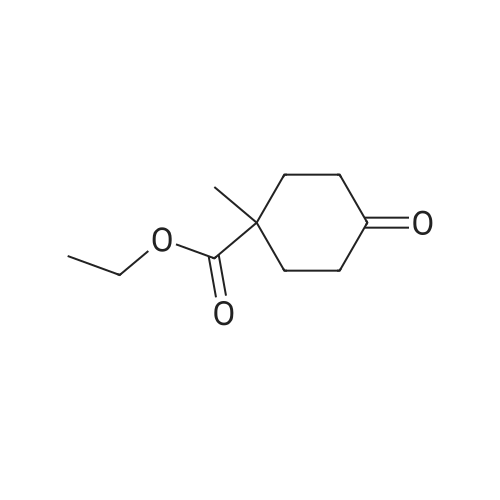
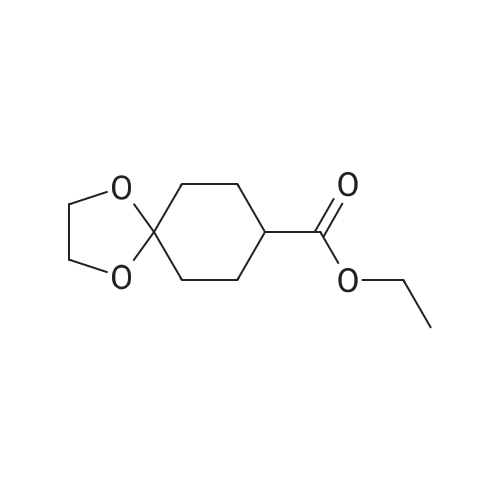
Ethyl 1-methyl-4-oxocyclohexane-1-carboxylate To a solution of ethyl 8-methyl-1,4-dioxaspiro[4.5]decane-8-carboxylate (1 equiv.) in acetone/H2O (0.5 M, 1/1) was added pTSA.H2O (1 equiv.). The mixture was refluxed overnight, then concentrated to remove acetone. The resulting solution was diluted with ethyl acetate, and the layers were separated. The organic phase was washed with saturated NaHCO3, dried over Na2SO4 and filtered. The filtrate was concentrated to afford ethyl 1-methyl-4-oxocyclohexanecarboxylate (67%). 1H NMR (400 MHz, CDCl3) delta ppm: 4.23 (q, J=7.2 Hz, 2H), 2.49-2.39 (m, 4H), 2.37-2.27 (m, 2H), 1.75-1.64 (m, 2H), 1.34-1.28 (m, 6H). |
|
With hydrogenchloride; water; In 1,4-dioxane; ethanol; at 20℃; for 64h;Product distribution / selectivity; |
Step B - Synthesis of ethyl l-methyl-4-oxocyclohexanecarboxylate (Int-6b) OC2009.701871lnt-6a lnt-6bIn a 50 mL round-bottom flask was mixed with ethyl 8-methyl-l,4- dioxaspiro[4.5]decane-8-carboxylate (Int-6a, 1.99 g, 8.72 mmol), EtOH (10 mL), H2O (5 mL), and 4N HCl in 1,4-dioxane (5 mL). The resulting solution was stirred at room temperature for 64 hours. At this time, the solvent was removed in vacuo and the residue was taken up in DCM. The suspension was washed with saturated NaHCtheta3(aq) and the organic layer was dried over Na2SO^ The resulting product was taken on without further purification. Yield = 1.00 g. |
|
With ammonium cerium (IV) nitrate; water; In acetonitrile; at 70℃; for 1h; |
The ketal compound (2.09 g, 9.17 mmol) was dissolved in CAN (100 mL) and water (50 mL). H4)2Ce(N03)6 (503 mg, 0.92 mmol) was added in water (50 mL) was added and the mixture was heated up to 70 C and stirred for 1 hour. After cooling down to room temperature, water (100 mL) was added and extracted with Et20 (100 mL x 3) and the organics was dried over Na2S04. After concentration, the crude was purified with column (0-30% EtOAc/Hexane) to give the product (1.72 g). |
|
With ammonium cerium (IV) nitrate; In water; acetonitrile; at 70℃; for 1h; |
The ketal compound (2.09 g, 9.17 mmol) was dissolved in CAN (100 mL) and water (50 mL). (NH )2Ce N03)6 (503 mg, 0.92 mmol) in water (50 mL) was added and the mixture was heated up to 70 C and stirred for 1 hour. After cooling down to room temperature, water (100 mL) was added and extracted with Et20 (100 mL x 3) and the organics was dried over Na2S04. After concentration, the crude was purified with column (0~30% EtOAc/Hexane) to give the product (1.72 g). |
|
With hydrogenchloride; In water; acetone; at 20℃; for 48h; |
Intermediate 66: ethyl l-methyl-4-oxocyclohexanecarboxylateTo a solution of ethyl 8-methyl-l,4-dioxaspiro[4.5]decane-8-carboxylate (2.0 g, 8.76 mmol) in aceton (60 ml) was added HC1 (2.5 M, 60 ml, 150 mmol) at romm temperature . After stirring at room temperature over 48 hours, the reaction mixture was poured into DCM , the organic layer was then separated and the aqueous was extracted with DCM, washed with brine, dried over Na2S04 , filtered and concentrated, and purified by MPLC (5-60%> EtOAc in hexane ) to provide ethyl l-methyl-4-oxocyclohexanecarboxylate as colorlee liquid (1.12 g). LC-MS (ES, m/z) CioHi603: 184; Found: 185 [M+H]+. Intermediate 67: ethyl 4-hydroxy-l-methylcyclohexanecarboxylateTo a solution of ethyl l-methyl-4-oxocyclohexanecarboxylate (7.02 g, 38.1 mmol) in methanol (15 ml) at 0C added sodium borohydride (0.721 g, 190.5 mmol) in small portions over 30 min. The reaction mixture aged for 1 hour. Then concentrated under vacuum and applied onto a silica gel column and eluted with ethyl acetate/hexane 10-100%. This resulted in 5.58 g (79%) of ethyl 4-hydroxy-l-methylcyclohexanecarboxylate (cis&trans mixture) as colorless oil. LC-MS (ES, m/z): Ci0Hi8O3: 186; Found: 187 [M+H]+. |
| 8.34 g |
With hydrogenchloride; In water; acetone; at 20℃; |
The compound (12.8 g) produced in Example 44 was dissolved in 100 mL of acetone, 50 mL of 2N hydrochloric acid was added while stirring at room temperature, and the mixture was stirred at that temperature overnight. After completion of the reaction, the solvent was distilled off under reduced pressure, 100 mL of tetrabutyl methyl ether was added, and extraction operation was performed. The resulting organic layer was sequentially washed with an aqueous saturated sodium bicarbonate solution and an aqueous saturated sodium chloride solution, and dried with magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain 9.7 g of the crude product. The crude product was purified by silica gel column chromatography (ethyl acetate/hexane=3%?25%) to obtain the title compound (8.34 g) having the following physical property values.; TLC: Rf 0.41 (hexane:ethyl acetate=4:1); 1H-NMR (CDCl3): delta 1.25-1.32 (m, 6H), 1.58-1.73 (m, 2H), 2.27-2.51 (m, 6H), 4.22 (q, J=7.2 Hz, 2H). |
| 1.12 g |
With hydrogenchloride; In water; acetone; at 20℃; |
Intermediate 35 Ethyl 1 -methyl-4-oxocvclohexanecarboxylate To a solution of ethyl 8-methyl-l,4-dioxaspiro[4.5]decane-8-carboxylate (2.0 g, 8.76 mmol) in aceton (60 ml) was added HC1 (2.5 M, 60 ml, 150 mmol) at rt . After stirred at rt over weekend, the reaction mixture was poured into DCM , the organic layer was then separated and the aqueous was extracted with DCM, washed with brine, dried over Na2S04 , filtered and concentrated, and purified by MPLC (5-60% EtOAc in hexane ) to provide ethyl l-methyl-4- oxocyclohexanecarboxylate as colorlee liquid (1.12 g). LC-MS (ES, m/z) C10H16O3: 184; Found: 185 [M+H]+ |
|
With sulfuric acid; In acetone; at 20℃; |
Step 3. Preparation of ethyl 1-methyl-4-oxocyclohexanecarboxylate (i-ld). The mixture of ethyl 8-methyl-l,4-dioxa-spiro[4.5]decane-8-carboxylate (i-lc) (2.0 g, 8.77 mmol) in Acetone (20 mL) and IN H2S04 (20 mL) was stirred at room temperature for overnight. The mixture was diluted with H20 (50 mL). The aqueous layer was extracted with DCM (3x30 mL). The combined organic layers were washed with brine (30 mL), dried over anhydrous Na2S04 and concentrated to get the desired product as a pale yellow oil. LCMS (ESI) calc'd for Ci0Hi6O3 [M+H]+: 185, found: 185. |
|
With sulfuric acid; In acetone; at 20℃; |
Step 3. Preparation of ethyl l-methyl-4-oxocyclohexanecarboxylate (i-ld).A mixture of ethyl 8-methyl-i ,4-dioxa-spiro [4.5] decane-8-carboxylate (i-l c) (2.0 g, 8.77 mmol) in acetone (20 mL) and iN H2504 (20 mL) was stirred at room temperature overnight. The mixture was diluted with H20 (50 mL). The aqueous layer was extracted with DCM (3x30 mL). The combined organic layers were washed with brine (30 mL), dried over anhydrous Na2504 and concentrated to obtain the desired product as a pale yellow oil. LCMS(ESI) calc?d for C10H1603 [M+H]b: 185, found: 185. |
|
With sulfuric acid; In water; acetone; at 20℃; |
Step 3. Preparation of ethyl 1-methyl-4-oxocyclohexanecarboxylate (i-1d) [0261] A mixture of ethyl 8-methyl-1,4-dioxa-spiro[4.5]decane-8-carboxylate (i-1c) (2.0 g, 8.77 mmol) in acetone (20 mL) and 1N H2SO4 (20 mL) was stirred at room temperature overnight. The mixture was diluted with H2O (50 mL). The aqueous layer was extracted with DCM (3×30 mL). The combined organic layers were washed with brine (30 mL), dried over anhydrous Na2SO4 and concentrated to obtain the desired product as a pale yellow oil. LCMS (ESI) calc'd for C10H16O3 [M+H]+: 185. found: 185. |
|
With hydrogenchloride; water; In acetone; at 20℃; for 18h; |
To a solution of compound D2-2 (8.4 g, 36.84 mmol) in acetone (100 mL) was added HCl (3 M in water* 50 mL) dropwise at room temperature, and the whole was stirred at room temperature for 18 h. The reaction mixture was quenched with water (100 mL) and extracted with EtOAc (2 x 25 mL). The combined organic layers were washed with water (100 mL), brine (100 mL), dried over anhydrous Na2S04 and concentrated under reduced pressure to provide compound D2-3 (6.3 g) as a light yellow oil. The crude product was used in the next step without purification. 1H NMR (CDC13, 400 MHz): delta 4.22 (q, J = 7.0 Hz, 2H), 2.47-2.38 (m, 4H), 2.34-2.30 (m, 2H), 1.72-1.64 (m, 2H), 1.31- 1.29 (m. 611). |
|
With ammonium cerium (IV) nitrate; In water; acetonitrile; at 70℃; for 2h; |
Step 3. Preparation of ethyl l-methyl-4-oxocvclohexanecarboxylate (i-23c) [00196] To a solution of ethyl 8-methyl-l,4-dioxaspiro[4.5]decane-8-carboxylate (7.3 g, 32.0 mmol) in MeCN (50 mL) and water (50 mL) was added eerie ammonium nitrate (2.11 g, 3.85 mmol). The mixture was heated to 70 C and stirred for 2 h. The reaction mixture was cooled to room temperature, and partitioned between water (50 mL) and EtOAc (50 mL). The aqueous layer was extracted with EtOAc (50 mL* 2). The combined organic layers were washed with brine (50 mL*2), dried over Na2SC>4, filtered and concentrated under reduced pressure to afford the title compound (5 g, 85%) as yellow oil, which was used for next step without further purification. |
|
With sulfuric acid; In acetone; at 20℃; for 14h; |
Into a 100-mL round-bottom flask, ethyl 8-methyl-1,4-dioxaspiro[4.5]decane-8- carboxylate (2.3 g, 10.08 mmol) was dissolved in acetone (20 mL). Then sulfuric acid (1M, 20 mL) was added. The resulting solution was stirred for 14 h at room temperature. The resulting solution was diluted with 50 ml of water and extracted with 3x50 ml of ethyl acetate. The organic layers were combined, dried over sodium sulfate, filtered and concentrated under vacuum. This afforded the title compound (1.8g, crude) as a yellow oil. MS: (ES, m/z): 185 [M+H]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping