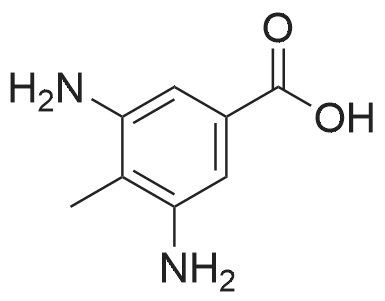
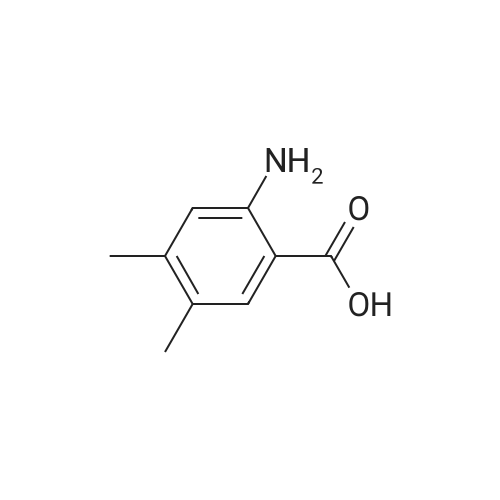
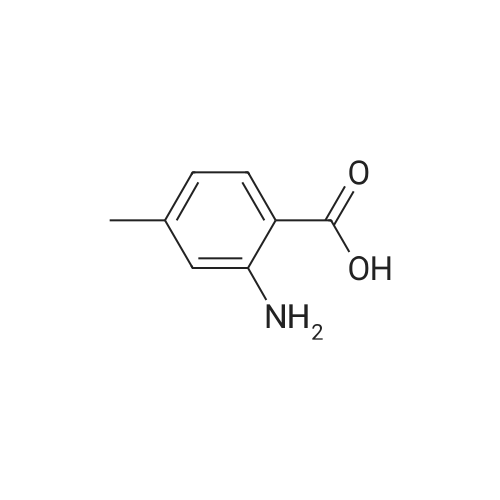
| 100% |
With sodium hydroxide; In water; at 20℃; for 18h; |
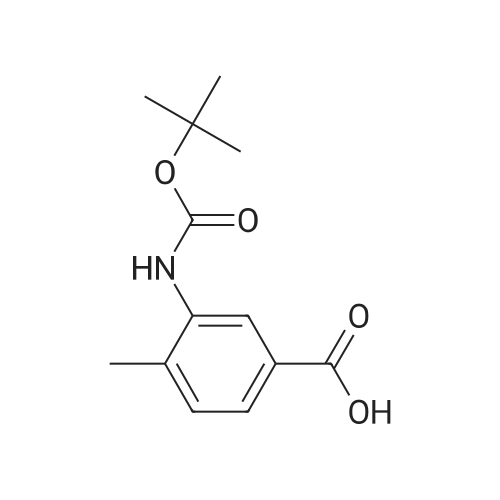
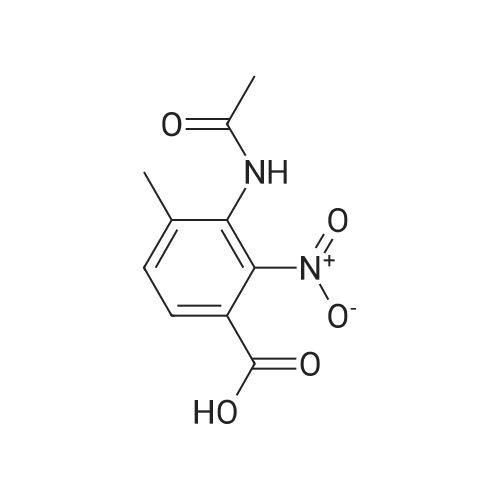
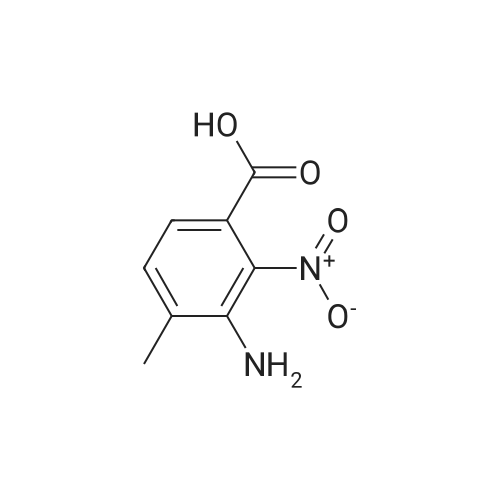
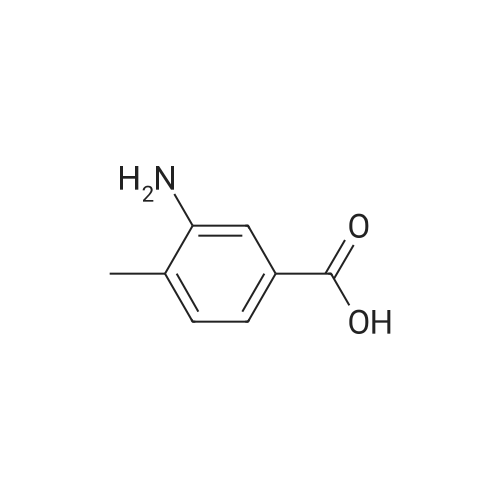
To a solution of 3-amino-4-methyl benzoic acid (2.0 g, 13.2 mmol) in water (25 mL) and 1N sodium hydroxide (25 mL) was added di-tert-butyl dicarbonate (4.3 g, 19.8 mmol) and the reaction was stirred for 18 hours at ambient temperature. The reaction was partitioned between ethyl acetate and 5% aqueous citric acid. The organic layer was washed with water and brine and dried over magnesium sulfate. The material was filtered and concentrated to provide the protected aniline as a pink solid (3.3 g, quantitative yield). |
| 91% |
In tetrahydrofuran; at 50℃; |
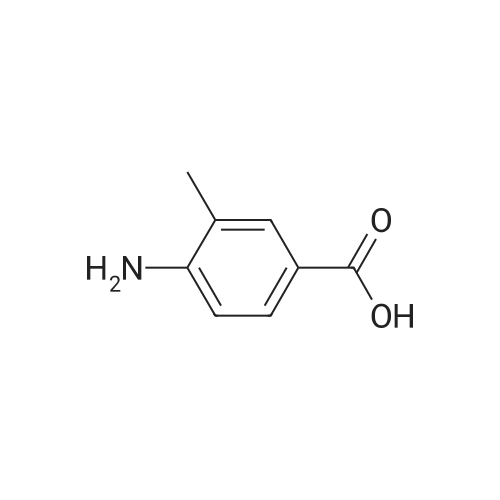
A mixture of commercially-available 4-amino-3-methylbenzoic acid (100 g, 0.66 mol) and di-tertbutyl dicarbonate (150 g, 0.68 mol) in THF (1000 mL) was slowly heated to 50C overnight. The resulting mixture was cooled to rt and the solvent was removed on a rotary evaporator. The resulting solids were triturated with hexanes and dried in vacuo to afford 151 g (91%) of the crude BOC-protected aniline intermediate as a light pink solid. To the above, light-pink solid was added 1- (3- dimethylaminopropyl) -3-ethylcarbodiimide hydrochloride (127 g, 0.66 mol), HOBt (90 g, 0.66 mol), and DMF (1000 ml), and the resulting mixture was stirred at rt for 30 minutes followed by addition of methoxyamine hydrochloride (55 g, 0.66 mol) in one portion. After stirring for 10 min, the mixture was cooled using an ice bath. Diisopropyl-ethylamine (250 ml, 1.4 mol) was added at such a rate so as to maintain the internal reaction temperature below 25C. After the addition was complete, the ice bath was removed and the reaction was stirred overnight at rt. The reaction mixture was partitioned between 0.5 L of water and 1.5 L of EtOAc and the resulting layers were separated. The aqueous portion was extracted with additional EtOAc (400 mL x 3), and the combined organic extracts were washed with, water (300 mL x 3), cold 0.5 N aqueous HC1 (400 mL x 2), and water (500 mL). The product was then extracted with cold 0.5 N aqueous NaOH (300 mL x 3) and the combined basic aqueous extracts were neutralized to pH = 8 by a slow addition of cold 0.5 N aqueous HC1. The resulting solid which precipitated was collected by filtration and washed with cold water.'The wet solid was decolorized in hot EtOH with active charcoal to give 106 g of white solid as the BOC-protected N-methoxyamide intermediate. To a slurry of the above solid (91 g, 0.32 mol) in 1,4-dioxane (400 mL) at rt was added a 4M solution of HC1 in dioxane (400 mL), and the resulting mixture was stirred at rt overnight. Diethyl ether (1000 mL) was added and the precipitated solid was collected by filtration and triturated with a hot EtOH/H20 mixture (4: 1 v/v). Drying the resulting solid in vacuo afforded 53 g of the pure hydrochloride salt (1D) as a white solid. 1H NMR (d6-DMSO) : 6 9.5-9. 9 (br. s, 1H), 7.75 (s, 1H), 7.55 (d, 1H), 7.36 (d, 1H), 3.70 (s, 3H), 2.38 (s, 3H). |
| 88% |
In tetrahydrofuran; at 50℃; |
A suspension of 3-amino-4-methylbenzoic acid (20.0 g, 132 mmol) and N-(tert-butoxycarbonyl)anhydride (30.0 g, 219 mmol) in THF (200 mL) was heated and stirred at 50 C overnight. The resulting mixture was cooled to rt. The solvent was evaporated in vacuo, and the crude product was recrystallized from EtOAc to afford 7 (29.1 g, 88 %) as a pink solid. 1H NMR (DMSO-d6): delta 12.8 (s, 1H), 8.66 (s, 1H), 7.97 (s, 1H), 7.59 (dd, J=2.0, 8.0Hz, 1H), 7.28 (d, J=8.0Hz, 1H), 3.25 (s, 3H), 1.47 (s, 9H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping