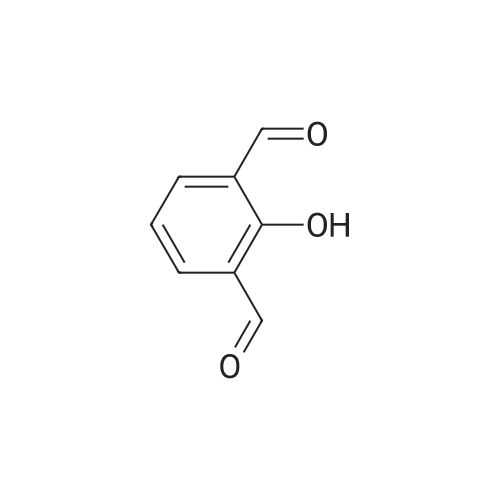
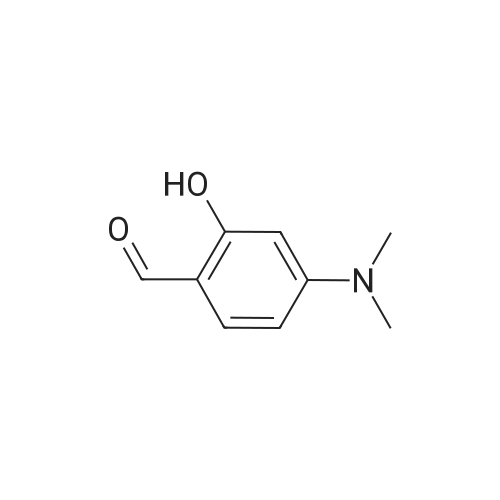
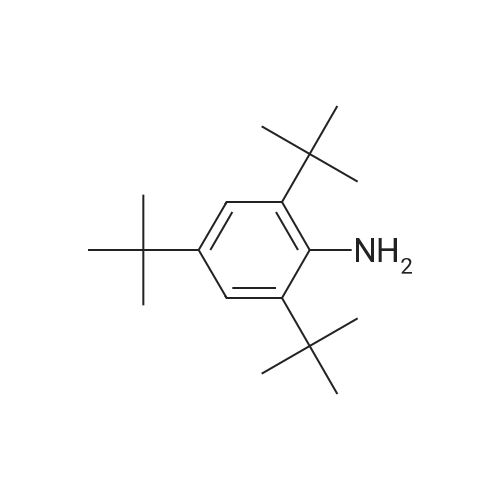
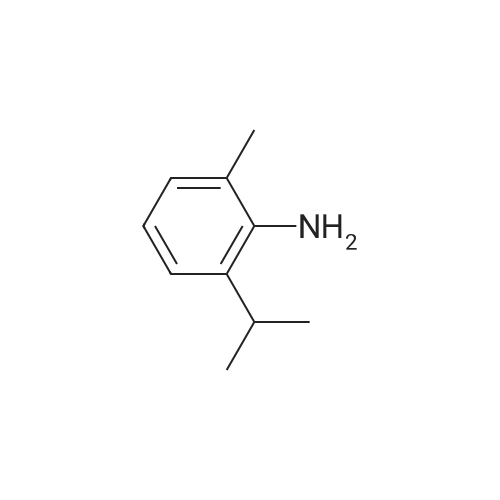
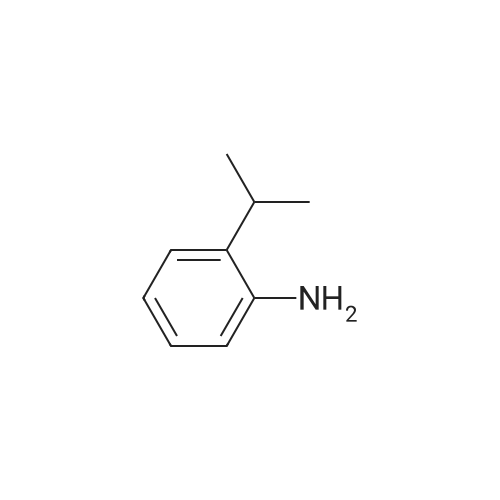
| 95% |
With tetra-N-butylammonium tribromide; In tetrahydrofuran; at 0℃; |
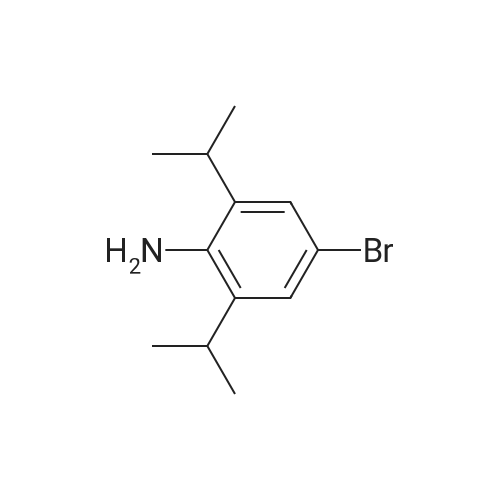
40.8 g (85 mmol) of tetra-n-butylammonium tribromide are added, at 0 C. and portionwise, to a solution of 15 g (85 mmol) of 2,6-diisopropylphenyl-amine in 200 ml of tetrahydrofuran. The medium is stirred for 2 h. It is then poured into a saturated aqueous solution of sodium thiosulphate and extracted with ethyl acetate. The organic phases are combined and washed with water. They are dried over sodium sulphate. The residue is purified by silica gel chromatography (80/20 heptane/ethyl acetate). 20.6 g of 4-bromo-2,6-diisopropylphenylamine are obtained in the form of a yellow oil (yield=95%). |
| 88% |
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0℃; for 1h; |
To a stirred solution of 2,6-diisopropylaniline (5.05 g, 28.4 mmol, 1.00 equiv) inN,Ndimethylformamide (70 mL) at 00 C was added a solution of NBS (5.05 g, 28.4 mmol, 1.00 equiv) in N,N-dimethylformamide (30 mL) dropwise over 60 mm. The reaction was stirred foranother hour at 0 C, at which time water (300 mL) was added. The resulting mixture was extracted with ethyl acetate (2 x 300 mL), and the combined organic layers were washed with saturated NH4C1 solution (3 x 100 mL) followed by water (100 mL) and dried over anhydrous sodium sulfate. Concentration of the solution under vacuum afforded 4-bromo-2,6- diisopropylaniline (6.5 g, 88% yield). LCMS (Method A): 256.1 [M+H], retention time 2.97mm. |
| 82% |
With tetra-N-butylammonium tribromide; In tetrahydrofuran; at 20℃; |
Preparative Example P16Step 1 : 4-Bromo-2,6-diisopropylaniline (PI 6a)To a solution of 2,6-diisopropylaniline (12.5 g, 70.6 mmol) in THF was added (C4H9)4NBr3 (47 g, 85 mmol) at rt and stirred overnight. Then sat. NaHS03 solution (30 mL) was added and the mixture was extracted with Et20 (3 x 250 mL). The organic layer was washed with brine, dried over Na2S04 and concentrated to give compound P16a (19 g, 82%). |
| 79% |
With bromine; In methanol; at 20℃; |
A solution of 2, [6-DIISOPROPYLANILINE] (2.0 mL, 11 mmol) in methanol (80 mL) was treated dropwise with bromine (543 muL, 10.6 mmol). The solution was stirred at room temperature for 15 min. Chloroform and 0.1 N [NAOH] were added and the organic layer was dried (Na2SO4), filtered and concentrated to an orange liquid. Kugelrohr distillation [(110 C @ ] 0.06 Torr) afforded a colorless liquid (2.22 g 79%): 1H NMR (400 MHz) 8 1.25 (d, J= 6.4 Hz, 12H), 2. 88 (m, 2H), 3.70 (brs, 2H), 7.11 (s, 2H) [; 13C NMR 8] 22.1, 27.9, 111.0, 125.6, 134.5, 139.3 ; Anal calcd for [C12HIGBRN] : C, 56.26 ; H, 7. 08 ; Br, 31.19 ; Found, C, 56.24 ; H, 7.07 ; Br, 30.98 |
| 72% |
With bromine; In methanol; dichloromethane; at 20℃; for 24h; |
A solution of Br2(2.1 ml, 41.0 mmol, 1.05 eq.) in CH2Cl2/MeOH (100 ml, 1:1 v/v)was added to a stirring solution of 2,6-diisopropylaniline (7.4 ml,39.0 mmol, 1.0 eq.) in CH2Cl2/MeOH (200 ml, 1:1 v/v) at r.t. over2 h. The orange-red solution was stirred for 1 day. The solvents were evaporated and the resultant pink solid was washed with PE and further recristallized from CH2Cl2/PE to give the title compound as light orange-red solid (7.8 g, 28.1 mmol, 72%).1H-NMR (300 MHz,CDCl3) [ppm]: 10.08 (s, 2H), 7.36 (s, 2H), 3.80-3.62 (m, 2H), 1.29(d, J = 6.7 Hz, 12H). The analytical data are in accordance with theliterature. |
| 65.7% |
With N-Bromosuccinimide; In acetonitrile; at 20℃; |
Into a 500-mL round-bottom flask, was placed 2,6-bis(propan-2-yl)aniline (10 g, 56.4 mmol) in ACN (200 mL), to the stirred solution was added NBS (11.0 g, 62.0 mmol). The resulting solution was stirred overnight at RT. The resulting mixture was concentrated undervacuum. The residue was eluted from silica gel with petroleum ether. This resulted in 9.5 g (65.7%) of the title compound as brown oil. MS-ESI: 256/258 [M+1]. |
| 48.8 g (42%) |
With potassium hydroxide; bromine; In water; acetic acid; |
4-Bromo-2,6-diisopropylaniline To a solution of 80.0 g (0.452 mol) of 2,6-diisopropylaniline in 2000 cm3 of glacial acetic acid, 23.3 ml (72.3 g, 0.452 mol) of bromine were added dropwise, while vigorously stirring, over 20 minutes. This mixture was stirred additionally for 2 hours at 40 C. The white precipitate that formed was filtered off, washed with 100 ml of acetic acid, and dried in air. The resulting white solid was added to a solution of 150 g of potassium hydroxide in 600 ml of water. This mixture was stirred for 30 minutes. The product was extracted with 3*100 ml of methyl-tert-butyl ether. The combined organic fractions were washed with Na2SO4 and evaporated to dryness. Fractional distillation gave a yellowish oil, b.p. 112-115 C./1 mm Hg. Yield 48.8 g (42%). Anal. calc. for Cl2H18BrN: C, 56.26; H, 7.08. Found: C, 56.11; H, 7.13. 1H NMR (CDCl3): delta 7.10 (s, 2H, 3,5-H), 3.69 (br.s, 2H, NH2), 2.86 (sept, J=6.8 Hz, 2H, CHMe2), 1.23 (d, J=6.8 Hz, 12H, CHMe2). 13C{1H}NMR (CDCl3): delta 139.3, 134.5, 125.6, 111.0, 27.9, 22.1. |
|
|
Reference Example 4 4-bromo-2,6-diisopropyl-N,N-dimethylaniline To a solution of 2,6-diisopropylaniline (25 g, 0.141 moL) in toluene (25 mL) was added dimethyl sulfoxide (12.1 g, 1.1 equivalents), and the mixture was heated to 90C. 48% Aqueous hydrobromic acid solution (26.1 g, 1.1 equivalents) was added dropwise to the mixture at the same temperature over 30 min. Then, the mixture was stirred at 86C for 3 hr and at 100C for 2 hr. Water (20 mL) was added to the mixture at 0C, and 1M aqueous sodium hydroxide solution (30 mL) was added dropwise to the mixture. The mixture was partitioned, and the organic layer was washed with 1M aqueous sodium hydroxide solution (50 mL). The organic layer was dried over anhydrous magnesium sulfate and filtered naturally, and the filtrate was concentrated under reduced pressure. The residue was distilled under reduced pressure to give 4-bromo-2,6-diisopropylaniline (29.4 g, pale-yellow liquid). |
| 540 g |
With N-Bromosuccinimide; In N,N-dimethyl-formamide; at 0 - 20℃; for 18h; |
Apparatus set-up:A 10 L 3-necked round-bottomed flask, equipped with a mechanical overhead stirrer, nitrogen inlet and exhaust.Experimental Procedure:1. 2, 6-Diisopropyl aniline (500 g, 2.8202 mol) was dissolved in dry N, N-dimethyl formamide.It was cooled to 0 C using an ice bath.2. N-Bromosuccinimide (501.9 g, 2.8202 mol) was added slowly portion wise.3. The reaction mixture was stirred at the room temperature for 18 h.4. After 18 h, TLC showed complete conversion of starting material.5. To the mixture, 10% NaHCO3 solution (4 L) was added and extracted with ethyl acetate (3 x2L).6. The combined organic phase was washed with water (1 L), brine (1 L), dried over sodium sulphate and concentrated.7. The crude product was purified by silica column chromatography using 8 % ethyl acetate in hexane as an eluent to get 540 g with 95.75 % HPLC purity. 1H-NMR (300 MHz, CDCl3): delta[ppm] 1.26 (d, J = 6.78 Hz, 12H), 2.84 - 2.93 (m, 2H), 3.72 (br, s, 2H), 7.12 (s, 2H). |
|
With bromine; In methanol; at 20℃; |
49 ml (152 g, 954 mmol) ofbromine was slowly (for 2 h) added to a stirred solutionof 169.7 g (957 mmol) of2,6-diisopropylaniline in 1500 ml of methanol. Theresulting dark-red solution was stirred overnight at room temperature, then most ofmethanol was removed under reduced pressure, and 1000 ml of dichloromethane was added to the residue. This solution was shaken with a cold solution of 140 g (2.5mol) of potassium hydroxide in 1100 ml of water. The organic layer was separated,and the aqueous one was extracted with 200 ml of dichloromethane. The combinedorganic extract was dried over K2C03, passed through a short layer of silica gel 60( 40-63 )liD), and evaporated in vacuum to give crude 4-bromo-2,6-diisopropylaniline (purity ca. 90%) as dark-red oil which was further used without an additionalpurification.1H NMR (CDCh): b 7.15 (s, 2H), 3.82 (br.s, 2H), 2.90 (sept, J = 6.9 Hz, 2H), 1.28(d, J = 6.9 Hz, 12H). 13CeH} NMR (CDCh,): b 139.15, 134.55, 125.65, 111.08,27.92, 22.16. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping