| 100% |
|
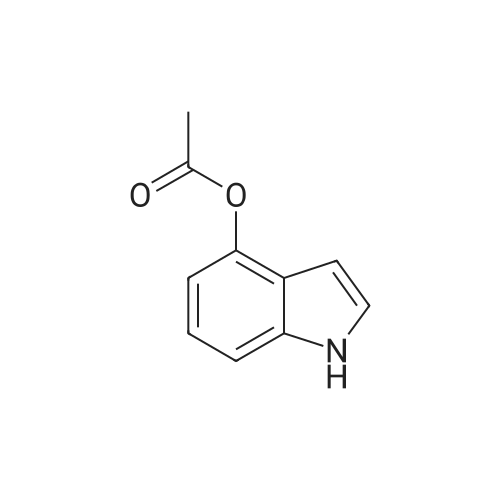
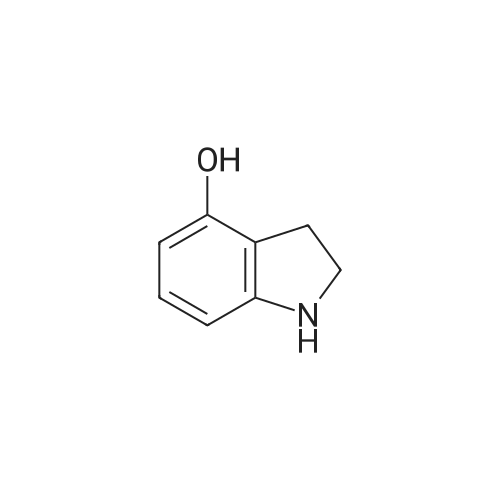
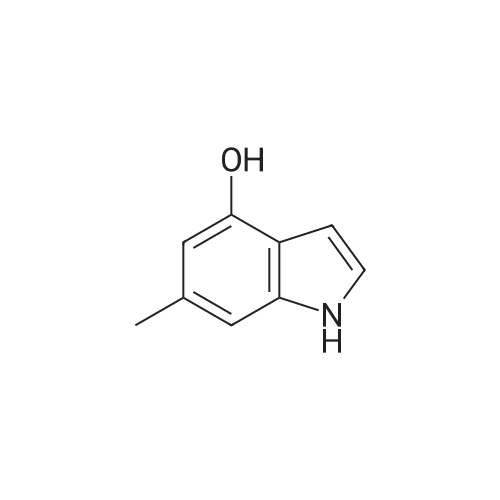
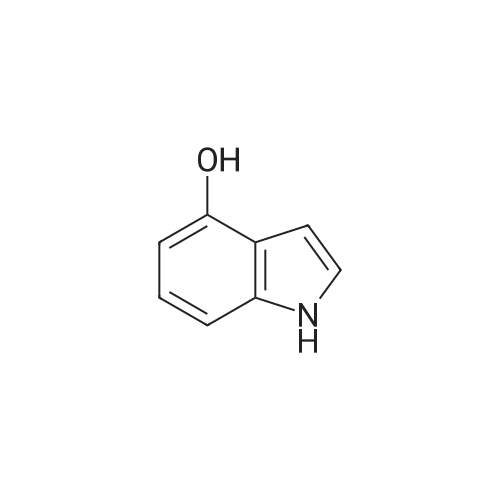
Synthesis of CDNI-GAB A.; [0068] Synthesis of compound 2b.; 4-Acetoxy-l-acetylindoline. A solution of 4-hydroxyindole (6.66 g, 50 mmol) in acetic acid (250 mL) was treated with NaBH3CN (9.42 g, 150 mmol) over 0.5 h, keeping the temperature at <15 C. The mixture was then stirred at room temperature for 1 h and water (5 mL) was added and the solvent evaporated. The residue was dissolved in EtOAc (150 mL) and washed with saturated aq. NaHCO3 and brine, dried and evaporated to give 4- hydroxyindoline as pale crystals (6.76 g. 100%); IH NMR (δ, CDCl3 DMSO-J6): 6.82 (IH, t, J= 8 Hz), 6.20 (IH, d, J= 8 Hz), 6.16 (IH, d, J= 8 Hz), 3.52 (2H, t, J= 8 Hz) and 2.90 (2H, d, J= 8 Hz). The crude indoline was dissolved in a mixture of acetic acid (50 mL) and acetic anhydride (50 mL) and heated under reflux for 1 h. The solution was diluted with water (10 mL) and the solvents evaporated. The residue was dissolved in EtOAc (150 mL) and washed with saturated aq. NaHCO3 and brine, dried and evaporated to give 4-acetoxy-l- acetylindoline as pale crystals (9.01 g, 82%), NMR (δ, 90 MHz): 8.07 (IH, d, J= 8 Hz), 7.19 (IH, t, J= 8 Hz), 6.72 (IH, d, J= 8 Hz), 4.05 (2H, t, J= 8 Hz), 3.03 (2H, t, J= 8 Hz) 2.28 (3H, s) and 2.19 (3H, s). |
| 100% |
With sodium cyanotrihydridoborate; In glacial acetic acid; at 15 - 20℃; for 1.5h; |
A solution of 4-hydroxyindole 26 (6.66 g, 50 mmol) in acetic acid (250 mL) was treated with NaBH3CN (9.42 g, 150 mmol) over 0.5 h, keeping the temperature at-15oC. The mixture was stirred at room temperature for 1 h and water (5 mL) was added and the solvent evaporated. The residue was dissolved in EtOAc (150 mL) and washed with saturated aq. NAHC03 and brine, dried and evaporated to give 4- hydroxyindoline as pale crystals (6. 76 g. 100%) ; 1H NMR: (90 MHz, CDC13 + DMSO-d6) # 6. 82 (1H, t, J = 8 Hz), 6.20 (1H, d, J = 8 Hz), 6.16 (1H, d, J = 8 Hz), 3.52 (2H, t, J = 8 Hz) and 2.90 (2H, d, J = 8 Hz). |
| 99% |
With sodium cyanotrihydridoborate; In glacial acetic acid; at 15 - 20℃; |
A solution of 4-hydroxyindole (0.16 g; 1.2 mmol) in AcOH (6 ml) was treated with NaBH3CN (0.23 g; 3.6 mmol), at rate keeping the temperature below 150C. The mixture was then stirred for 1 h at room temperature and H2O (0.4 ml) was added and solvents removed in vacuo. The residue was diluted to 15 ml with EtOAc, washed with 5% NaHCO3, brine, dried over anhydrous MgSO4, filtered and filtrate evaporated under reduced pressure to give the title compound (0.16 g; 99%) as creamy solid. 1 H-NMR (CDCI3 ) 2.93 (tr, 2H, J = 8.4 Hz); 3.58 (tr, 2H, J = 8.4 Hz); 3.74 (broad S, 2H); 6.17 (d, 1 H, J = 8 Hz); 6.26 (d, 1 H, J = 7.72 Hz); 6.88 (tr, 1 H, J = 7.85 Hz). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping