|
With hydrogen sulfide; ammonia; |
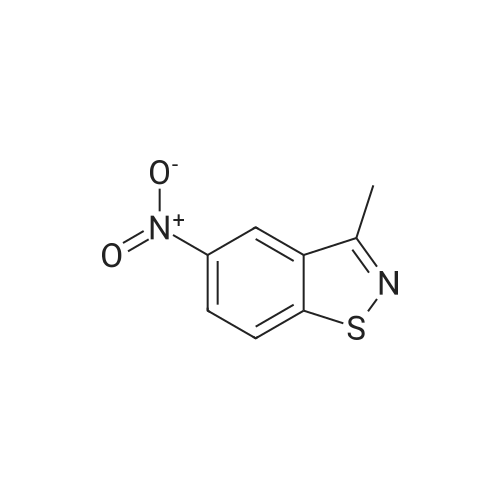
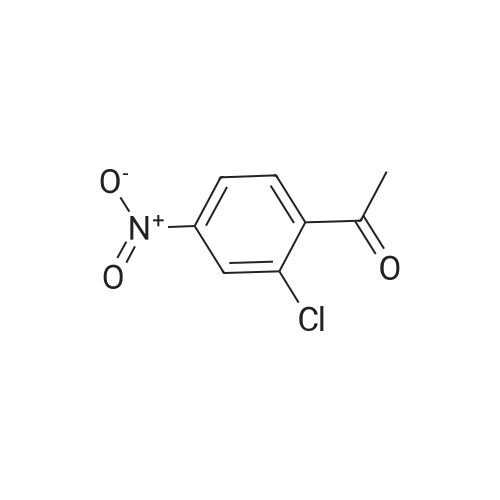
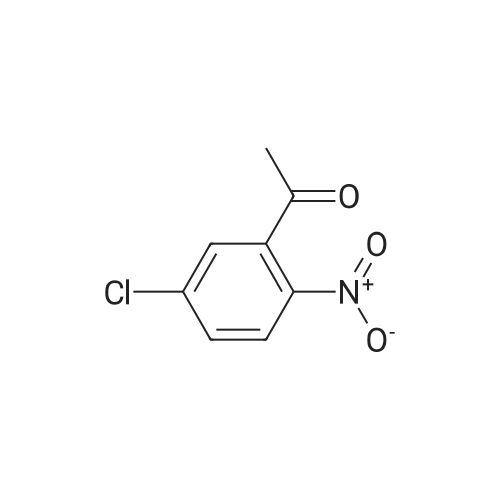
EXAMPLE 3 Preparation of 3-Methyl-5-nitro-1,2-benzisothiazole Ammonia (45 g, 2,642 mmol) is bubbled into methanol at -40 C in a steel bomb. Sulfur (30.5 g, 95.0 mmol) and 2'-chloro-5'-nitroacetophenone (19 g, 95.0 mmol) are then added. The bomb is sealed and heated at about 90 C overnight. After cooling, the reaction mixture is removed from the bomb and concentrated in vacuoto obtain a residue. The residue is diluted with methylene chloride, passed through a plug of silica gel and concentrated in vacuoto give the title product as an orange solid (12.0 g) which is identified by NMR spectral analyses. |
|
With hydrogen sulfide; ammonia; |
EXAMPLE 5 Preparation of 3-Methyl-5-nitro-1,2-benzisothiazole Ammonia (45 g, 2,642 mmol) is bubbled into methanol at -40 C. in a steel bomb. Sulfur (30.5 g, 95.0 mmol) and 2'-chloro-5'-nitroacetophenone (19 g, 95.0 mmol) are then added. The bomb is sealed and heated at about 90 C. overnight. After cooling, the reaction mixture is removed from the bomb and concentrated in vacuo to obtain a residue. The residue is diluted with methylene chloride, passed through a plug of silica gel and concentrated in vacuo to give the title product as an orange solid (12.0 g) which is identified by NMR spectral analyses. |
|
With octasulfur; ammonia; In methanol; at -40 - 90℃;Sealed bomb; |
REFERENCE EXAMPLE 7 Preparation of 3-Methyl-5-nitro-1,2-benzisothiazole ammonia (45 g, 2,642 mmol) is bubbled into methanol at -40 C in a steel bomb.. sulfur (30.5 g, 95.0 mmol) and 2'-chloro-5'-nitroacetophenone (19 g, 95.0 mmol) are then added.. The bomb is sealed and heated at about 90 C overnight.. After cooling, the reaction mixture is removed from the bomb and concentrated in vacuo to obtain a residue.. The residue is diluted with methylene chloride, passed through a plug of silica gel and concentrated in vacuo to give the title product as an orange solid (12.0 g) which is identified by NMR spectral analyses. Using essentially the same procedure, the following compounds are obtained: |
|
With hydrogen sulfide; ammonia; |
EXAMPLE 35 Preparation of 3-Methyl-5-nitro-1,2-benzisothiazole Ammonia (45 g, 2,642 mmol) is bubbled into methanol at -40 C. in a steel bomb. Sulfur (30.5 g, 95.0 mmol) and 2'-chloro-5'-nitroacetophenone (19 g, 95.0 mmol) are then added. The bomb is sealed and heated at about 90 C. overnight. After cooling, the reaction mixture is removed from the bomb and concentrated in vacuo to obtain a residue. The residue is diluted with methylene chloride, passed through a plug of silica gel and concentrated in vacuo to give the title product as an orange solid (12.0 g) which is identified by NMR spectral analyses. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping