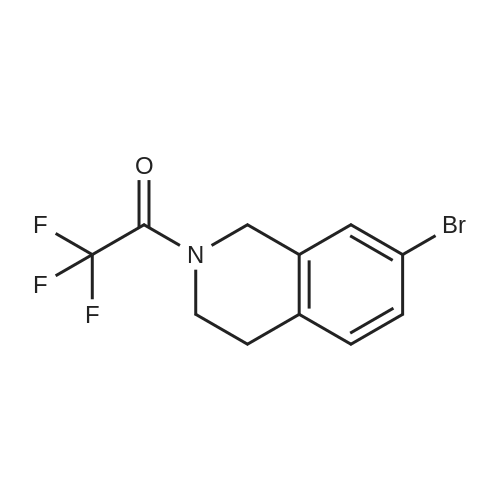
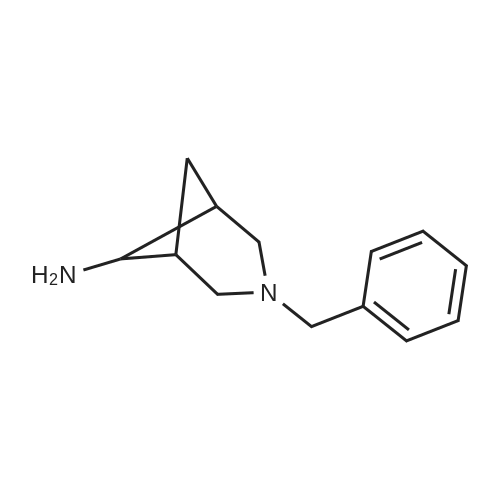
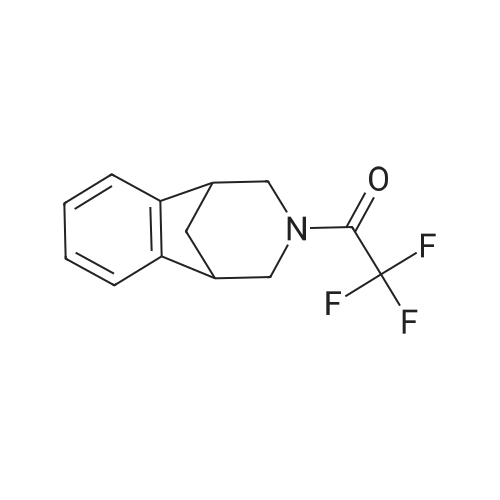
| 94% |
With nitric acid; In trifluoroacetic acid; at 0℃; for 2.16667h; |
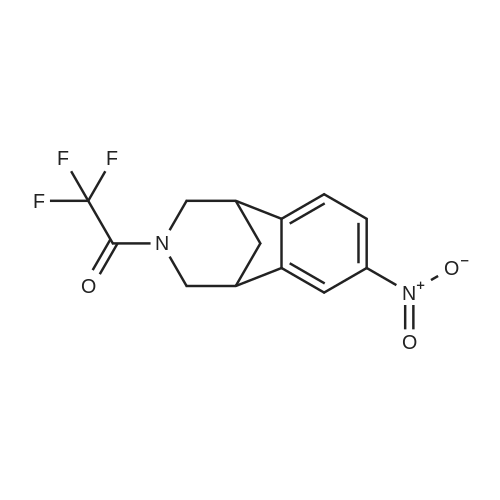
A solution of 1-(10-Aza-tricyclo[6.3.1.02l7]dodeca-2(7),3,5-trien- 10-yl)-2,2,2-trifluoro-ethanone (49.9g, 196 mmol) (4) (see O'Donnell et al., JOC, 2004 (69,7), 5756-59 and International Publication Nos. WO 04/063164 and WO 99/35131) and in trifluoroacetic acid (TFA) (100 mL) was cooled in an acetone/ice bath and treated drop-wise with fuming HNO3 over 10 minutes. The resultant reaction mixture was stirred for 1 hour as the ice bath temperature increased to 0C and then for an additional 1 hour. The ice bath was removed, excess NO2 was removed under a stream of nitrogen, and TFA was removed <n="84"/>under reduced pressure. The resultant residue was poured into 300 ml of ice water and extracted with 3 x 200 ml CH2CI2. The combined organic phases were washed with saturated NaCI (1 x 100 ml) and saturated NaHCO3. (1 x 100 ml). The organic phase was dried over MgSO4 and passed through a 200 g plug of silica gel (230-400 mesh) eluting with CH2CI2 (2000 ml). The resultant eluent was concentrated under reduce pressure to provide C17 as a pale yellow solid (55.4 g, 184 mmol, 94% yield). 1H NMR (400 MHz, DMSO-D6) delta ppm 2.1 (d, (d, J=4.6 Hz, 2 H) 3.7 (m, 1 H) 3.8 (m, 1 H) 4.1 (d, J=12.9 Hz, 1 H) 7.5 (t, J=8.5 Hz, 1 H) 8.1 (d, J=7.9 Hz, 1 H) 8.2 (dd, J=10.8, 2.1 Hz, 1 H) ppm. |
| 78% |
With trifluorormethanesulfonic acid; nitric acid; In dichloromethane; |
B 1-(4-Nitro-10-aza-tricyclo[6.3.1.02,7]dodeca-2(7),3,5-trien-10-yl)-2,2,2-trifluoro-ethanone (Based on the method described by Coon, C. L.; Blucher, W. G.; Hill, M. E. J. Org. Chem. 1973, 25, 4243.) To a solution of trifluoromethanesulfonic acid (2.4 ml, 13.7 mmol) in CH2Cl2 (10 ml) stirred at 0 C. was slowly added nitric acid (0.58 ml, 27.4 mmol) generating a white precipitate. After 10 minutes the resulting mixture was cooled to -78 C. and treated with <strong>[230615-51-7]1-(10-aza-tricyclo[6.3.1.02,7]dodeca-2(7),3,5-trien-10-yl)-2,2,2-trifluoro-ethanone</strong> (3.5 g, 13.7 mmol) in CH2Cl2 (15 ml) dropwise from an addition funnel over 5 minutes. The reaction was stirred at -78 C. for 30 minutes then warmed to 0 C. for 1 hour. The reaction mixture was poured into a vigorously stirred ice (100 g). The layers were separated and the aqueous layer extracted with CH2Cl2 (3*30 ml). The organic layer was combined and washed with H2O (3 *30 ml). The combined organic layer was washed with saturated aqueous NaHCO3 solution (20 mL) and H2O (20 mL) then dried through a cotton plug and concentrated to give an orange oil that solidified on standing (4.2 g). Chromatography yielded pure product as a crystalline solid (3.2 g, 78%). (TLC 30% ethyl acetate/hexanes Rf 0.23). 1H NMR (400 MHz, CDCl3) delta8.12 (br d, J=8.0 Hz, 1H), 8.08 (br s, 1H), 7.37 (br d, J=8.0 Hz, 1H), 4.38 (br d, J=12.6 Hz, 1H), 3.94 (br d, J=12.6 Hz, 1H), 3.59 (br d, J=12.6 Hz, 1H), 3.43-3.35 (m, 2H), 3.18 (br d, J=12.6 Hz, 1H), 2.48 (m, 1H), 2.07 (d, J=10.8 Hz, 1H). GCMS m/e 300 (M+). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping