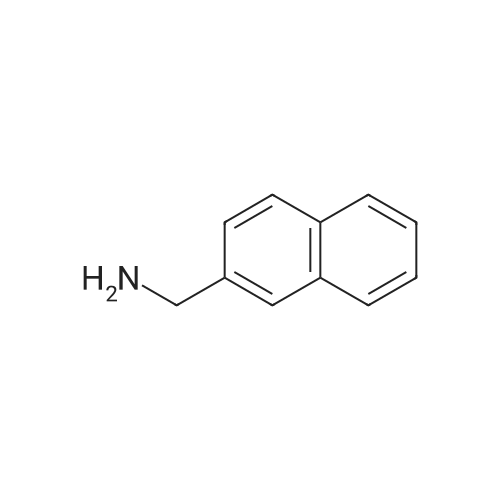
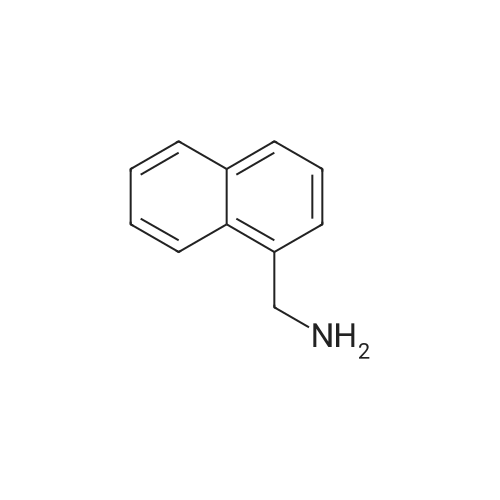
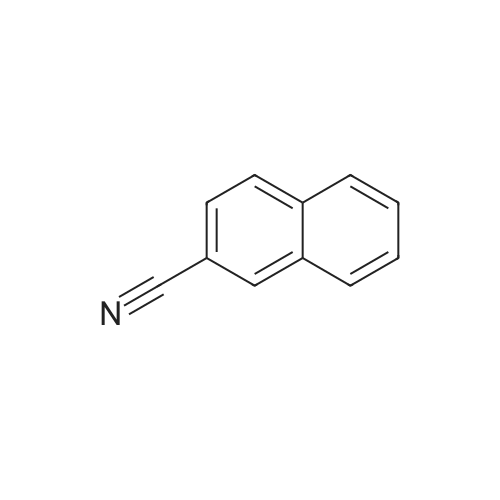
| 58% |
With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 - 20℃; |
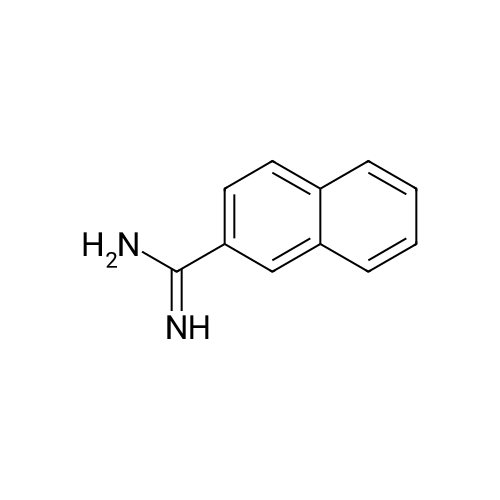
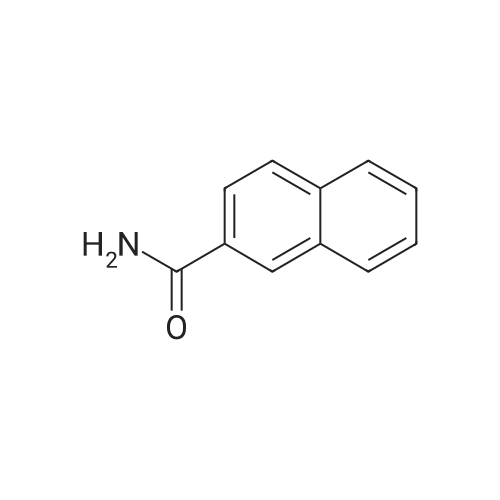
Naphthalene-2-carboxylic acid amide (0.8 g, 1 eq, 4.678 mmol) was dissolved in THF (80 mL) and the solution was cooled down to 0-50C. A 1.0 M solution of Lithium Aluminium Hydride (LAH) in THF (1.42 g, 8.0 eq, 37.0 mmol) was added drop-wise at 0-50C. The reaction mixture was stirred at RT overnight. After completion of the reaction (confirmed by TLC), ethyl acetate at 0-50C was slowly added to quench the excess LAH in reaction mixture followed by the addition of saturated sodium sulphate solution (2 mL). The reaction mass was filtered through a hy-flow bed and the filtrate was concentrated in vacuo to afford the crude product.The crude product was purified by column chromatography using neutral silica gel of 60- 120 mesh size. A gradient of 3-4 percent methanol in DCM was used to elute the title compound (0.43 g, 58percent). |
| 56% |
|
Compound 27 (1.00 g, 5.8 mmol) in THF (20 mL) was added slowly to a solution of LAH (1.76 g, 46.4 mmol) in THF (45 mL) at 0° C. The solution was allowed to warm to room temperature and the reaction was stirred overnight. The reaction was cooled to 0° C. and quenched with H2O. The solids were filtered from the solution through celite and washed with hot THF. The filtrate was concentrated and the residue was dissolved in EtOAc (80 mL) and washed with 1 M HCl (3.x.30 mL). The aqueous layer was basified with 6 M NaOH to a pH of 12 and the precipitate was extracted with EtOAc (3.x.30 mL). The resulting organic solution was washed with brine (40 mL), dried with Na2SO4 and filtered. Concentration afforded a slightly yellow solid (510 mg, 56percent yield). m.p. 55-56° C. 1H NMR (CDCl3) delta 7.80 (3H, ArH), 7.72 (s, 1H, ArH), 7.43 (m, 3H, ArH), 4.00 (s, 2H, ArCH2). 13C NMR (CDCl3) delta 140.6, 133.5, 132.5, 128.2, 127.7, 126.1, 125.8, 125.5, 125.1, 46.6. IR (KBr) vmax cm-1: 3362, 3291, 3050, 2915, 1950, 1596, 1507, 1358, 1273. GC: r.t.=8.97 min. EI-MS m/z (percent) 157 (83, M+), 156 (100), 141 (15), 129 (49), 128 (40), 127 (24), 115 (10). |
|
With dimethylsulfide borane complex; In tetrahydrofuran; at 0 - 60℃; for 3h; |
To a solution of the crude amide obtained in the above step (1) inTHF (100 ml), BMS (27.5 ml, 0.2904 mol) was slowly added at 0 °C. Theresulted reaction mixture was heated to 60 °C for 3hrs, quenched with 5percent HCIat 0 °C, extracted with EA and washed with 5percent HCI. The aqueous layerswere combined and basified with 1N NaOH, and again extracted with EA.The organic layers were combined and concentrated to give the title compound(13 g) as white solid.TLC System 1 : MC/MeOH =90:10 v/v Rf=0.231H-NMR (300 MHz, CDCI3) 5 ppm: 4.07(s, 2H), 7.48(m, 3H),7.79(m, 4H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping