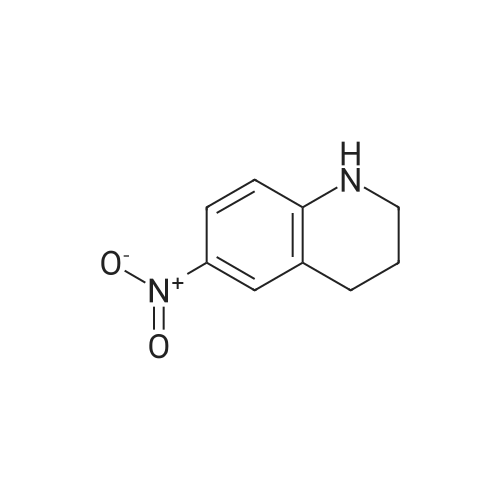
| 29% |
|
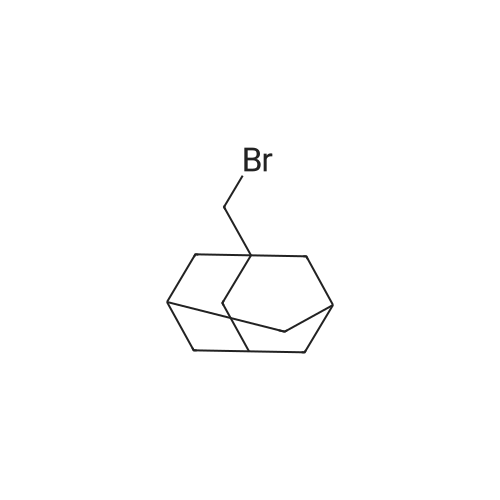
6-Nitro-3,4-dihydroquinolin-2(1H)-one (2.00 g, 10.41 mmol) was dissolved under argon in abs. N,N-dimethylformamide and admixed with fine potassium carbonate powder (4.31 g, 31.22 mmol). After stirring at room temperature for 5 min, 1-bromomethyladamantane (3.10 g, 13.53 mmol) and potassium iodide (0.86 g, 5.20 mmol) were added. The resulting reaction mixture was stirred at 120 C. for 5 h and, after cooling to room temperature, water and ethyl acetate were added. The aqueous phase was then repeatedly extracted with ethyl acetate. The combined organic phases were dried over magnesium sulfate, filtered and concentrated under reduced pressure. By column chromatography purification of the crude product obtained (ethyl acetate/heptane gradient), 1-(adamantan-1-ylmethyl)-6-nitro-3,4-dihydroquinolin-2(1H)-one (1.01 g, 29% of theory) was isolated as a colorless solid. In the next step, 1-(adamantan-1-ylmethyl)-6-nitro-3,4-dihydroquinolin-2(1H)-one (1.01 g, 2.97 mmol) was added together with tin(II) chloride dihydrate (2.68 g, 11.87 mmol) to abs. ethanol (30 mL) and the mixture was stirred under argon at a temperature of 50 C. for 3 h. After cooling to room temperature, the reaction mixture was poured into ice-water and then adjusted to pH 12 using aqueous NaOH. The aqueous phase was then repeatedly extracted with ethyl acetate. The combined organic phases were dried over magnesium sulfate, filtered and concentrated under reduced pressure. By column chromatography purification of the crude product obtained (ethyl acetate/heptane gradient), 1-(adamantan-1-ylmethyl)-6-amino-3,4-dihydroquinolin-2(1H)-one (0.86 g, 94% of theory) was isolated as a colorless solid. 1H-NMR (400 MHz, CDCl3 δ, ppm) 6.94 (d, 1H), 6.55-6.52 (m, 2H), 3.79-3-30 (br. s, 2H, NH), 3.54 (m, 2H), 2.79 (m, 2H), 2.59 (m, 2H), 1.89 (m, 3H), 1.66 (m, 2H), 1.63 (m, 2H), 1.58 (m, 4H), 1.49 (m, 4H). 1-(Adamantan-1-ylmethyl)-6-amino-3,4-dihydroquinolin-2(1H)-one (230 mg, 0.74 mmol) was dissolved together with (4-fluorophenyl)methanesulfonyl chloride (232 mg, 1.11 mmol) in abs. acetonitrile (7 mL) in a baked-out round-bottom flask under argon, then pyridine (0.12 mL, 1.48 mmol) was added and the mixture was stirred at room temperature for 12 h. The reaction mixture was then concentrated under reduced pressure, the remaining residue was admixed with dil. HCl and dichloromethane, and the aqueous phase was extracted repeatedly with dichloromethane. The combined organic phases were dried over magnesium sulfate, filtered and concentrated under reduced pressure. By column chromatography purification of the crude product obtained (ethyl acetate/heptane gradient), N-[1-(adamantan-1-ylmethyl)-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl]-1-(4-fluorophenyl)methanesulfonamide (210 mg, 59% of theory) was isolated as a colorless solid. 1H-NMR (400 MHz, CDCl3 δ, ppm) 7.29 (m, 2H), 7.10-7.02 (m, 3H), 6.98 (m, 1H), 6.87 (m, 1H), 6.06 (s, 1H, NH), 4.33 (s, 2H), 2.85 (m, 2H), 2.63 (m, 2H), 1.91 (m, 3H), 1.67 (m, 3H), 1.62-1.47 (m, 9H), 1.28 (m, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping