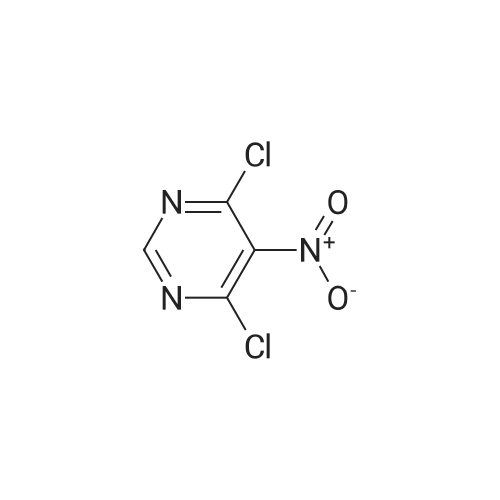
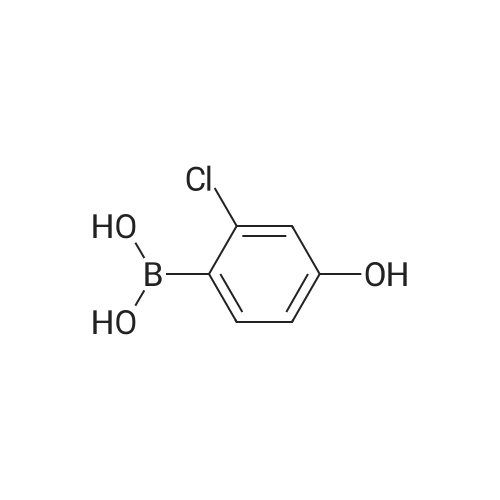
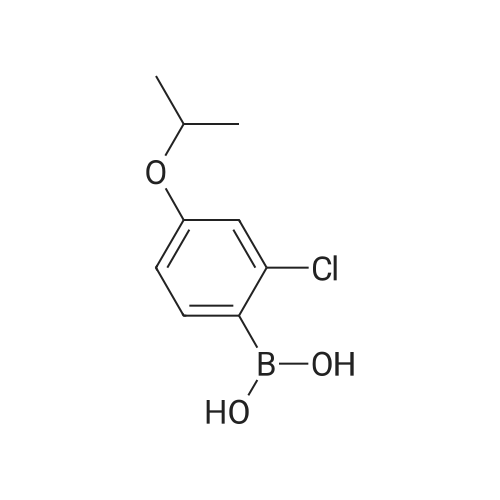
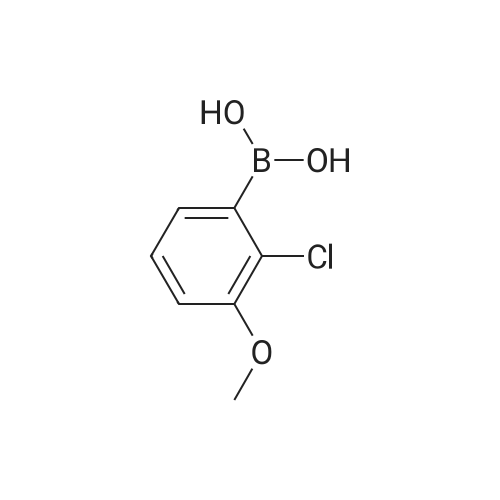
|
|
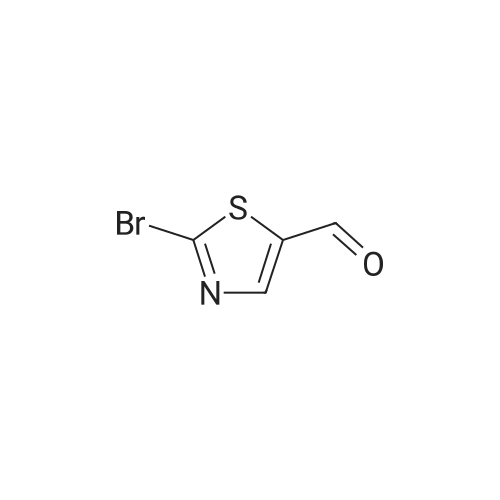
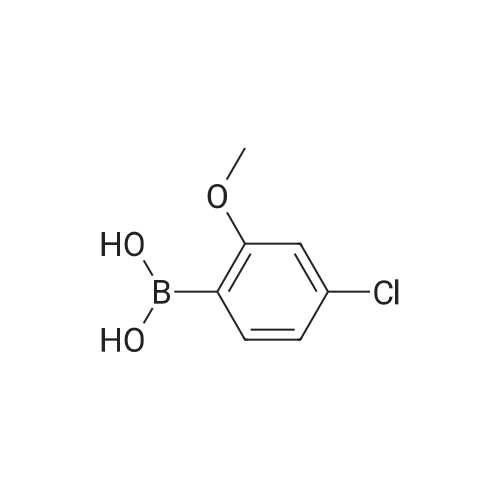
EXAMPLE 47; The mixture of <strong>[219735-99-6]2-chloro-4-methoxyphenyl boronic acid</strong> (372 mg), 2-bromo-5- formylthiazole(576 mg), sodium bicarbonate (6 mL, 1 M), dioxane (6 mL) and palladium tetrakistriphenylphosphine (30 mg) was heated at 1000C for 4 hours. The mixture was filtered through celite and diluted with ethyl acetate (100 mL) and washed with water (100 mL) followed by brine (50 mL). The organic fraction was dried with sodium sulfate and concentrated in vacuo to give the coupled product as a brown solid. To a solution of trimethyl phosphonoacetate (146 mg) in 5 mL of THF was added n-butyllithium (0.59 mL, 1.6 M in hexane) at O0C. The resulting solution was stirred at this temperature for 30 min. To this solution was added a THF solution (5 mL) of the above intermediate aldehyde (170 mg). The mixture was slowly warmed to rt and stirred for 2 hours. After quenching the mixture was water, the mixture was extracted with ethyl acetate, dried with sodium sulfate and concentrated in vacuo to give the enoate as a brown oily solid. To this enoate (83 mg) was added 5 mL of EPO <DP n="53"/>THF:MeOH:water (3:1:1) followed by LiOH (2 mL, 1 M). The mixture was stirred at rt for 5 hours. After acidified with concentrated HCl until pH = 4, the slurry was extracted with 30% isopropanol in chloroform, dried with sodium sulfate and concentrated in vacuo to give the enoic acid as a yellow solid. To this enoic acid (100 mg) was added toluene (5 mL) and thionyl chloride (2 mL). The mixture was heated to reflux for 1 hour and the solvent was distilled off under reduced pressure. The residue was taken up with toluene (5 mL) and to it was added anthranilic acid methyl ester (74 mg). The resulting mixture was heated to reflux for additional 1 hour. The solvent was removed and the residue was taken up with DMSO (6 mL). Only part of solid dissolved, the remaining solid was filtered and LC-MS showed it was mainly the desired compound, which was taken up with methanol (18 mL). To this mixture was added tosyl hydrazide (500 mg). The mixture was heated at reflux. After one day, an additional 300 mg of tosyl hydrazide was added. After two and a half days, the resulting mixture was concentrated and dissolved in acetone. The solution was directly purified by biotage (5%-25% ethyl acetate in petroleum ether) to give the anthranilide methyl ester as an oily solid. This methyl ester was dissolved in 5 mL of THF:MeOH: water (3:1:1) followed by LiOH (3 mL, 1 M). The mixture was stirred at rt for 4 hours. After Gilson purification, the acid was obtained as a white solid. To this methyl ether derivative was added 5 mL of dichloromethane and 0.23 mL of borontribromide (0.23 mL, IN in dichloromefhane) at O0C. After stirring at RT for 2h, the reaction was quenched by water at O0C. The mixture was concentrated in vacuo and then dissolved by DMSO. The DMSO solution was purified by Gilson to give Example 47 as a white solid. IH NMR (acetone-d6, 500 MHz) delta 11.42 (s, IH), 8.56 (d, IH), 8.07 (d, IH), 7.77 (d, IH), 7.70 (s, IH), 7.56 (t, IH), 7.15 (t, IH), 6.95 (d, IH), 6.84 (dd, IH), 3.34 (t, 2H), 2.88 (t, 2H); LCMS m/z 401 (M-I), 403 (M++l). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping