Alternatived Products of [ 2177-47-1 ]
Product Details of [ 2177-47-1 ]
| CAS No. : | 2177-47-1 |
MDL No. : | MFCD00274253 |
| Formula : |
C10H10
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | YSAXEHWHSLANOM-UHFFFAOYSA-N |
| M.W : |
130.19
|
Pubchem ID : | 16587 |
| Synonyms : |
|
Chemical Name : | 2-Methyl-1H-indene |
Application In Synthesis of [ 2177-47-1 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 2177-47-1 ]
- 1
-
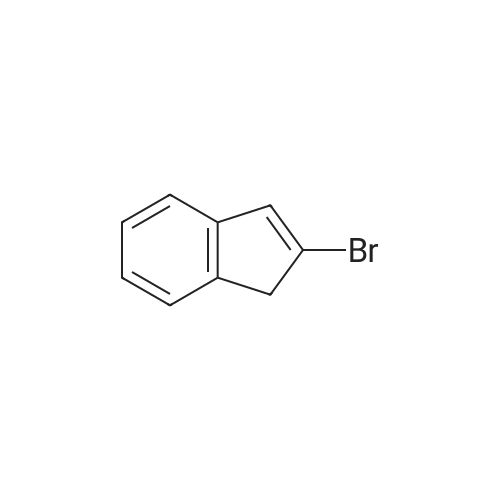 [ 10485-09-3 ]
[ 10485-09-3 ]

-
[3-(dimethylamino)propyl]dimethyl aluminium(III)
[ No CAS ]

-
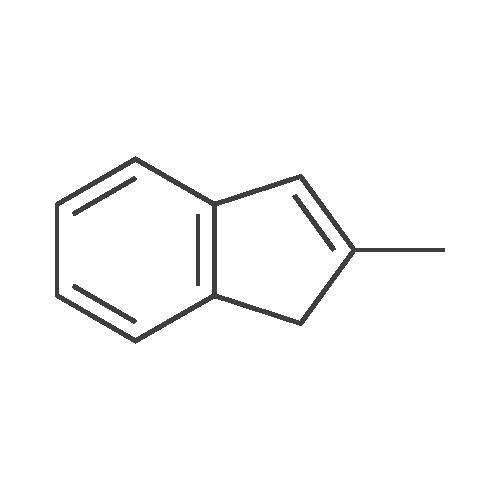 [ 2177-47-1 ]
[ 2177-47-1 ]

-
 [ 70063-93-3 ]
[ 70063-93-3 ]

-
 [ 95-13-6 ]
[ 95-13-6 ]
- 2
-
 [ 21906-31-0 ]
[ 21906-31-0 ]

-
 [ 2177-47-1 ]
[ 2177-47-1 ]
- 3
-
 [ 10485-09-3 ]
[ 10485-09-3 ]

-
 [ 2177-47-1 ]
[ 2177-47-1 ]
| Yield | Reaction Conditions | Operation in experiment |
|
Ni(dppp)Cl2; In diethyl ether; |
Preparation of 2-Methylindene via 2-Bromoindene 2-Bromoindene (24.4 g, 0.125 moles) and Ni(dppp)Cl2 (0.925 g, 1.71*10-3 moles) (dppp=1,3-bis(diphenyl-phosphino)propane) were stirred in diethylether (200 mL) at -78 C. under a nitrogen atmosphere as methylMgBr (0.150 moles, 50.00 mL of 3.0 M solution in diethylether) was added. The dry-ice bath was then immediately removed and the reaction mixture allowed to warm to room temperature. The reaction mixture started off as a heterogeneous brick-red color and then turned to a homogeneous yellow/gold solution. After an hour of stirring in this state an exotherm occurred which resulted in some refluxing of the ether in the flask. The solution then turned back to the heterogeneous brick-red mixture. Total stirring time for the mixture was 3 hours following the removal of the ice-bath after which time GC analysis showed that the conversion of <strong>[10485-09-3]2-bromoindene</strong> to 2-methylindene was substantially quantitative. After the reaction period the mixture was poured onto ice and then extracted with 1 M HCL (1*100 mL) and 1 M NaHCO3 (1*100 mL) and then dried with MgSO4 and filtered. |
- 4
-
 [ 2177-47-1 ]
[ 2177-47-1 ]

-
 [ 10485-09-3 ]
[ 10485-09-3 ]

-
 [ 75-78-5 ]
[ 75-78-5 ]

-
C21H22Si
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 87% |
|
In a fully dried, argon purged 200 mL reactor,Charge 1.95 g (8 0.1 mmol) of magnesium pieces,The mixture was vigorously stirred for 30 minutes while heating under reduced pressure.After cooling to room temperature,A piece of iodine and 25 mL of tetrahydrofuran were charged and stirred.Add slowly a diluted solution of 3.90 g (20.0 mmol) of <strong>[10485-09-3]2-bromoindene</strong> in 20 mL of tetrahydrofuran,The mixture was heated to reflux for 3 hours in an oil bath at 80 C.The reaction solution is diluted with 12.0 mL of dimethylsilyldichloride.(100 mmol) in 10 mL diluted solution of n-hexaneAdd slowly under -78 C cooling,Stirring was continued for 18 hours while returning to room temperature.After distilling off the solvent of the reaction solution and unreacted dimethylsilyl dichloride, 20 mL of tetrahydrofuran was added to the residue,1, 3-Dimethyl-2-imidazolidinone1.90 mL(20.2 mmol) was added. Dry enough,In a 100 mL reactor purged with argon,2.60 g (20. 0 mmol) of 2-methylindene,Charge 30 mL of tetrahydrofuran,n-butyllithium solution12. 2 mL (hexane solution,Add 1.64 M, 20.0 mmol),Stir at room temperature for 2 hours. This solution isAdd dropwise to the previously diluted reaction residue diluted solution cooled to -78 C,Stirring was continued for 3 hours while slowly returning to room temperature.Add saturated aqueous ammonium chloride solution,Extract the solubles with n-hexane,The obtained fraction is washed with saturated saline,It was dried over anhydrous magnesium sulfate.After filtering the magnesium sulfate,The residue obtained by distilling off the filtrate is purified by silica gel column chromatography,Target object shown by the following formula (A-1L)(Hereinafter referred to as Compound (A-1 L)) was obtained as an isomer mixture of 5.24 g (yield 87%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping










