| 87% |
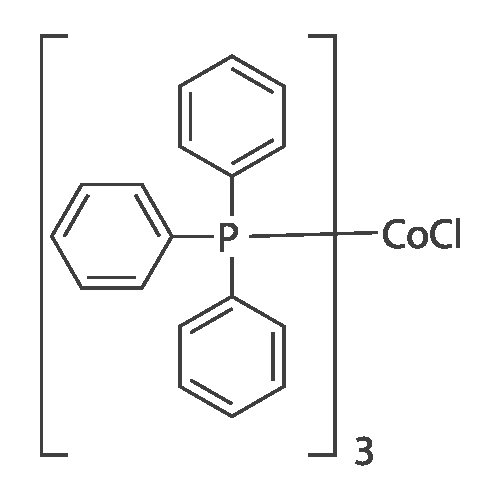
With triethylamine; lithium chloride;palladium diacetate; In N,N-dimethyl-formamide; at 100℃; for 3h; |
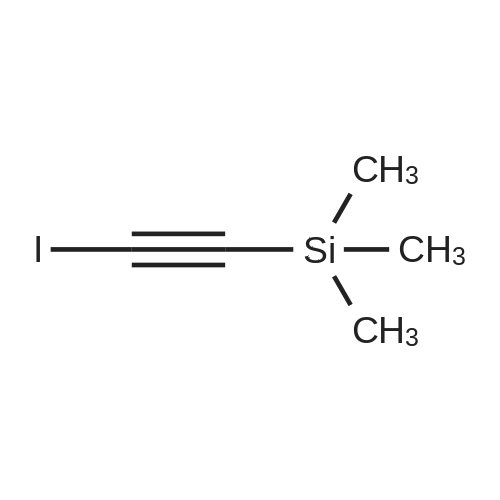
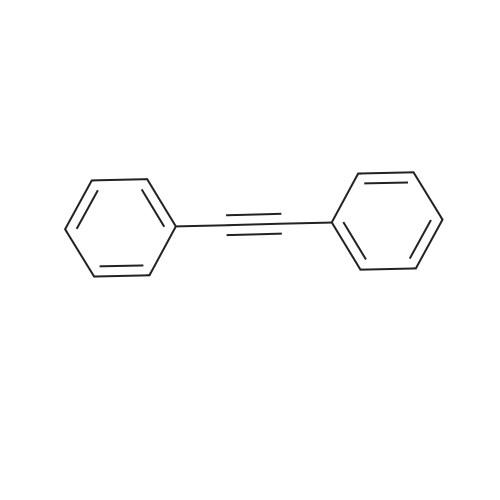
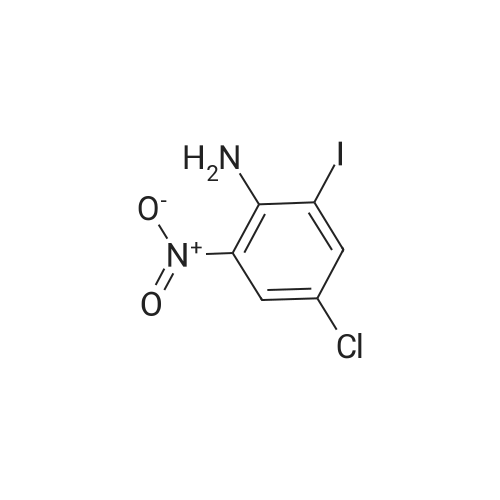
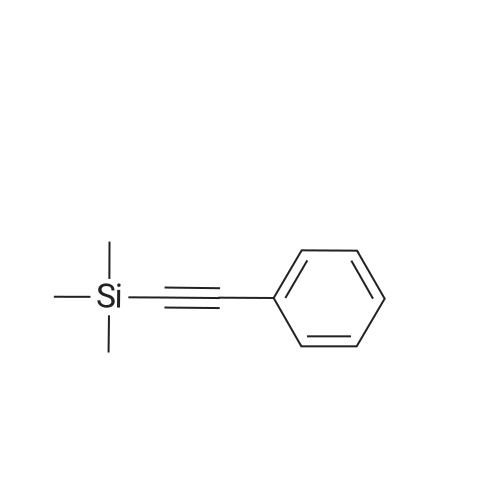
<strong>[123158-75-8]2-Amino-5-chloro-3-nitro-phenyliodide</strong> (1.5 g, 4.90 mmol) prepared in <n="51"/>Preparation 19 and l-phenyl-2-trimethylsilylacetylene (4.3 g, 24.50 mmol) were dissolved in DMF (50 rnL). Palladium acetate (0.11 g, 0.5 mmol), lithium chloride (0.21 g, 4.90 mmol) and triethylamine (2.48 g, 24.50 mmol) were added thereto, and the mixture was stirred under heating for 3 h to 100C . After completion of the reaction, water was added to the reaction mixture, which was then extracted with ethyl acetate, washed with saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, and filtered. The solvent was removed under reduced pressure, and the residue was purified by column chromatography to give the title compound (1.05 g, Yield 87%). 1H-NMR (400HMz, CDCl3); delta 9.78(br s, IH), 8.15(s, IH), 7.78(s, IH),7.38~7.48(m, 5H), 0.26(s, 9H) |
| 87% |
With triethylamine; lithium chloride;palladium diacetate; In N,N-dimethyl-formamide; at 100℃; for 3h; |
Preparation 41: 5-Chloro-3-phenyl-7-nitro-1H-indoleStep A: 5-Chloro-7-nitro-3-phenyl-2-trimethylsilyl-1H-indole; <strong>[123158-75-8]2-Amino-5-chloro-3-nitro-phenyliodide</strong> (1.5 g, 4.90 mmol) prepared in Preparation 19 and 1-phenyl-2-trimethylsilylacetylene (4.3 g, 24.50 mmol) were dissolved in DMF (50 mL). Palladium acetate (0.11 g, 0.5 mmol), lithium chloride (0.21 g, 4.90 mmol) and triethylamine (2.48 g, 24.50 mmol) were added thereto, and the mixture was stirred under heating for 3 h to 100 C. After completion of the reaction, water was added to the reaction mixture, which was then extracted with ethyl acetate, washed with saturated aqueous sodium chloride solution, dried over anhydrous magnesium sulfate, and filtered. The solvent was removed under reduced pressure, and the residue was purified by column chromatography to give the title compound (1.05 g, Yield 87%).1H-NMR (400 MHz, CDCl3); delta 9.78(br s, 1H), 8.15(s, 1H), 7.78(s, 1H), 7.387.48(m, 5H), 0.26(s, 9H) |
| 87% |
With triethylamine; lithium chloride;palladium diacetate; In N,N-dimethyl-formamide; at 100℃; for 3h; |
(Step 2)<strong>[123158-75-8]2-Amino-5-chloro-3-nitro-phenyliodide</strong> (1.5 g, 4.90 mmol) obtained in Step 1 and 1-phenyl-2-trimethylsilylacetylene (4.3 g, 24.50 mmol) were dissolved in DMF (50 mL), and thereto palladium acetate (0.11 g, 0.5 mmol), lithium chloride (0.21 g, 4.90 mmol) and triethylamine (2.48 g, 24.50 mmol) were added. The mixture was heated under stirring for 3 hours at 100 C. At the end of reaction, added water and extracted with ethylacetate. The extract was washed with saturated sodium chloride solution, dried over anhydrous magnesium sulfate and filtered. The solvent was removed under reduced pressure and the residue was separated by column chromatography to give 5-chloro-7-nitro-3-phenyl-2-trimethylsilyl-1H-indole (1.05 g, Yield 87%).1H-NMR (400 MHz, CDCl3); delta 9.78 (br s, 1H), 8.15 (s, 1H), 7.78 (s, 1H), 7.387.48 (m, 5H), 0.26 (s, 9H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping