|
|
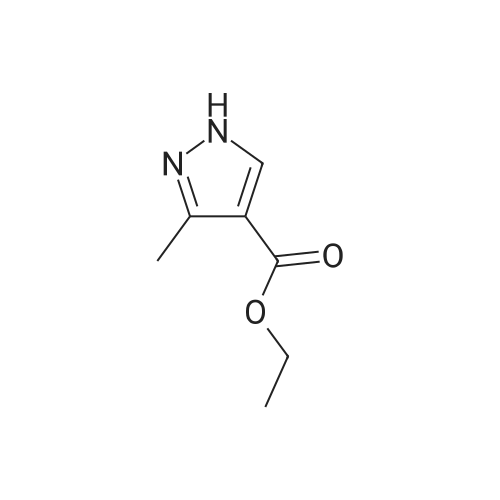
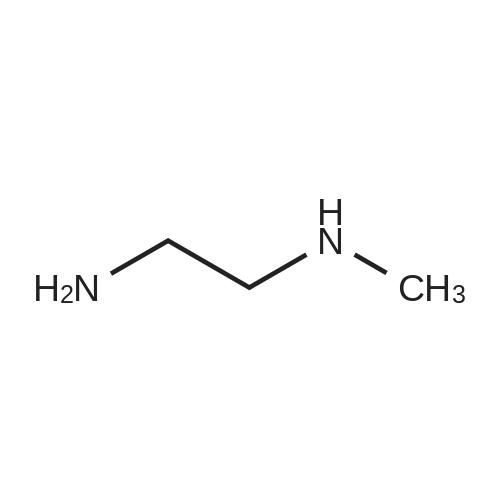
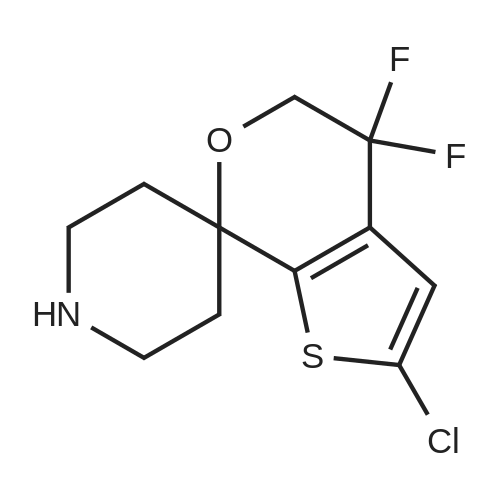
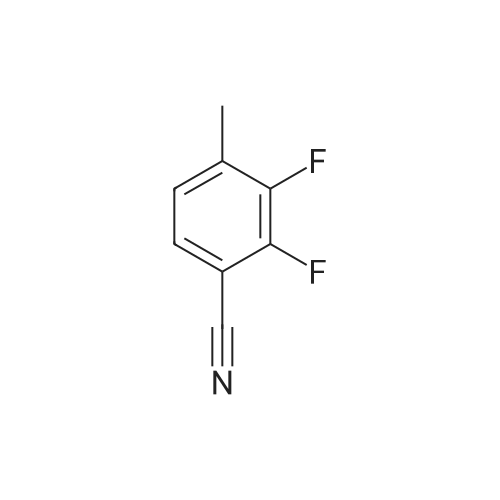
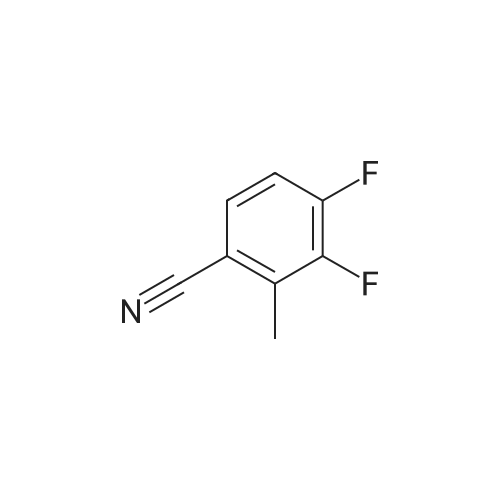
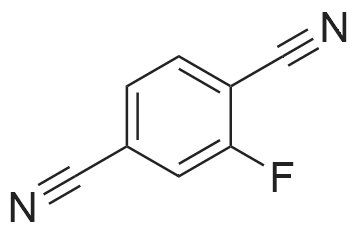
A mixture of 3 -methyl- lH-pyrazole-4-carboxylic acid ethyl ester (1.25 g, 8.1 1 mmol), potassium carbonate (1.68 g, 12.16 mmol), 2,3-difluorobenzonitrile (1.08 mL, 9.73 mmol) in dimethylformamide (12 mL) is heated at 100C with the aid of a magnetic stirred. After 2.5 hr. the reaction mixture is treated with water and extracted with ethyl acetate. The organic layer is decanted, washed with brine, dried over magnesium sulfate and the solvent evaporated under reduced pressure to give 2.3 g of l-(2-cyano-6-fluoro- phenyl)-3 -methyl- lH-pyrazole-4-carboxylic acid ethyl ester (this compound is contaminated with the other pyrazole regioisomer in a ratio 75:25). MS (m/z): 274 (M+1). A capped vial is charged with ethyl l-(2-cyano-6-fluoro-phenyl)-3-methyl- pyrazole-4-carboxylate (1.97 g, 7.21 mmol) (contaminated with the other pyrazole regioisomer in a ratio of 75:25, 1,2-ethanediamine, N-methyl- (6 mL, 68.02 mmol) and phosphorus pentasulfide (229 mg, 1,01 mmol) and the mixture is stirred at 1 10C for 30 min and then allow to reach rt. Solvent is evaporated in vacuo and the residue purified by normal phase Isco chromatography using dichloromethane/2M ammonia in methanol from 95/5 to 85/15 as eluent to yield 2.1 1 g of ethyl l-[2-fluoro-6-(l-methyl-4,5- dihydroimidazol-2-yl)phenyl]-3-methyl-pyrazole-4-carboxylate (contaminated with the other pyrazole regioisomer in a ratio 75:25). MS (m/z): 331 (M+1). Potassium permanganate (1.58 g, 10 mmmol) and montmorillonite K- 10 (3.16 g) are grounded together in a mortar until a fine homogeneous powder is obtained.KMn04-montmorillonite K-10 (3.2 g, 6.78 mmol) is added portionwise to a solution of ethyl l-[2-fluoro-6-(l-methyl-4,5-dihydroimidazol-2-yl)phenyl]-3-methyl-pyrazole-4- carboxylate (1.12 g, 3.39 mmol) (contaminated with the other pyrazole regioisomer in a ratio 75:25) in acetonitrile (84.76 mL, 1.62 moles). The mixture is stirred at room temperature for 6.5 hr. and more KMn04-montmorillonite K-10 (0.8 g, 1.69 mmol) is added portionwise and the mixture stirred at room temperature overnight. Ethanol is added and stirred for additional 20 min. Then the reaction mixture is filtered through a short pad of celite and the solid material is washed with acetonitrile. The solvent is evaporated under reduced pressure and the crude mixture is purified normal phase Isco chromatography using ethyl acetate as eluent to yield 518 mg of l-[2-fluoro-6-(l- methylimidazol-2-yl)phenyl]-3-methyl-pyrazole-4-carboxylate (contaminated with the other pyrazole regioisomer in a ratio 75:25). MS (m/z): 329 (M+1). 4. Gamma 1 - r2-Fruoro-6-C 1 -methylimidazol-2-yl phenyl1-3 -methyl-pyrazol-4-yllmethanolThis compound is essentially prepared as described in Preparation 29 by using ethyl l-[2-fluoro-6-(l-methylimidazol-2-yl)phenyl]-3-methyl-pyrazole-4-carboxylate (contaminated with the other pyrazole regioisomer in a ratio 75:25) in 99% yield. MS (m/z): 287 (M+l).5. l-r2-Fluoro-6-(l-methylimidazol-2-yl phenyl1-3-methyl-pyrazole-4-carbaldehvdeThe following compound is essentially prepared as described in Preparation 30 by using [l-[2-fluoro-6-(l-methylimidazol-2-yl)phenyl]-3-methyl-pyrazol-4-yl]methanol (contaminated with the other pyrazole regioisomer in a ratio 75:25). Residue is purified by normal phase Isco chromatography using ethyl acetate as eluent to give 64% yield of the title compound (contaminated with the other pyrazole regioisomer in a ratio 75:25). MS (m/z): 285 (M+l). To a screw-cap test tube containing a mixture of l-[2-fluoro-6-(l- methylimidazol-2-yl)phenyl]-3-methyl-pyrazole-4-carbaldehyde (288 mg, 1.01 mmol) (contaminated with the other pyrazole regioisomer in a ratio 75:25) and 2-chloro-4,4- difluoro-spiro[5H-thieno[2,3-c]pyran-7,4'-piperidine] (31 1.72 mg, 1.1 1 mmol) in 1,2- dichloroethane (3 mL) is stirred at room temperature for 1 hr. and then sodium triacetoxyborohydride (429.41 mg, 2.03 mmol) is added. The reaction tube is sealed and stirred at room temperature for 18 hr. with the aid of a magnetic stirrer. Then, the reaction is quenched by addition of sodium bicarbonate saturated solution and the compound is extracted with ethyl acetate. The organic layer is separated, dried over magnesium sulfate and the solvent removed under reduced pressure. The compound is purified by supercritical fluid chromatography using AD-H as stationary phase to provide 230 mg (41%) of the title compound as white solid. MS (m/z): 548 (M+l). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping