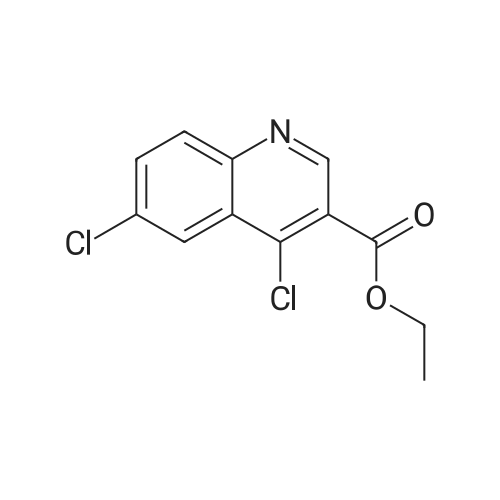
| 90% |
With oxalyl dichloride; N,N-dimethyl-formamide; In dichloromethane; at 35 - 40℃; for 6.5h; |
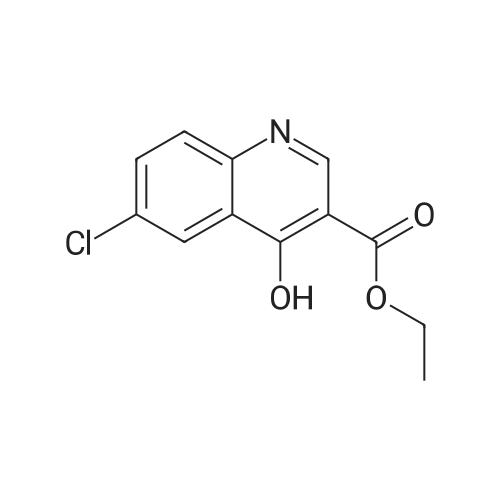
A mixture of ethyl-6-chloro-4-hydroxyquinoline-3-carboxylate DK-I-34-1 (85.1 g, 338.1 mmol), N,N- dimethylformamide (1.0 mL, 12.9 mmol), and dichloromethane (640 mL) was heated to 35- 40oC. Oxalyl chloride (47.2 g, 371.9 mmol) was added dropwise to the reaction mixture over 30 min. The reaction mixture was then heated for 6 h at reflux (38-40 oC). The resulting pale yellow solution was then cooled to 20-25 oC. The reaction mixture was then neutralized by slowly adding a 25% solution of potassium carbonate (75 g) in water (300 mL). The layers were then separated and the aqueous layers extracted with dichloromethane (200 mL). The combined organic layers were then washed with a 25% solution of potassium carbonate (50 g) in water (200 mL). The combined organic layers were then dried over magnesium sulfate. The solvents were then removed by evaporation on a rotovap and the product residue was slurried with hexanes (200 mL). The solid product was then filtered and washed twice with hexanes (50 mL x 2). The solid was dried to afford the product as an off- white solid DK-I-35-1 (81.9 g, 90%): 1H NMR (300 MHz, DMSO) delta 9.13 (s, 1H), 8.30 (d, J = 2.2 Hz, 1H), 8.14 (d, J = 9.0 Hz, 1H), 7.97 (dd, J = 9.0, 2.3 Hz, 1H), 4.44 (q, J = 7.1 Hz, 2H), 1.39 (t, J = 7.1 Hz, 3H); 13C NMR (75 MHz, DMSO) delta 164.01, 150.53, 147.73, 141.04, 134.30, 133.34, 132.20, 126.53, 124.37, 124.08, 62.59; HRMS m/z calculated for C12H10Cl2NO2 (M+H)+ 270.0088 found 270.10. |
| 82.52% |
With trichlorophosphate; at 110℃; for 2h; |
6-chloro-4-hydroxy- quinoline-3-carboxyiate (60, 2 g, 7.95 mmol) in phosphorus oxychloride (1.22 g, 7.95 mmol, 15 ml) and the reaction mixture was heated to 110C for 2 hours. The reaction mixture was cooled to room temperature and concentrated under reduced pressure. The crude product was diluted with water (20 mL) and the product was extracted with ethyl acetate (2x 100 mL). The combined organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to yield ethyl 4,6-dichloroquinoline-3-carboxylate (61, 1 9 g, 6 56 mmol, 82.52% yield) as yellow solid LCMS (ES+): m/z 272 [M + i i | |
|
With thionyl chloride; In water; |
(a) 4,6-Dichloroquinoline-3-carboxylic acid, ethyl ester A mixture of 45.2 g. of 6-chloro-4-hydroxyquinoline-3-carboxylic acid, ethyl ester (0.18 mol.) and 250 ml. of thionyl chloride is refluxed for 20 hours. The excess thionyl chloride is then removed in vacuo, the residue treated with 200 ml. of water and the ester is extracted with ether. After washing the ethereal extract twice with water, it is dried with Na2 SO4 and the solvent distilled off. The residual 4,6-dichloroquinoline-3-carboxylic acid, ethyl ester is triturated with petroleum ether (40-60), filtered and dried. Yield: 45.3 g. (93%); m.p. 87-88. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping