| 98% |
|
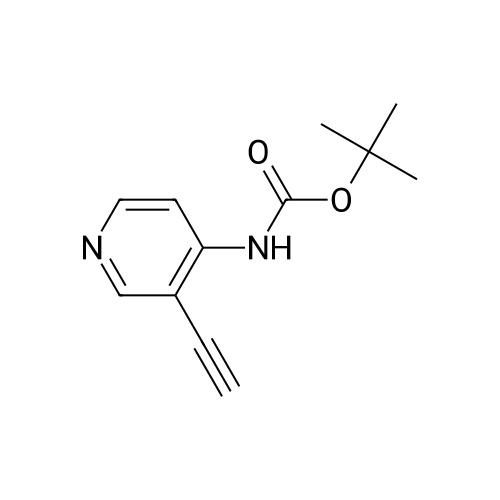
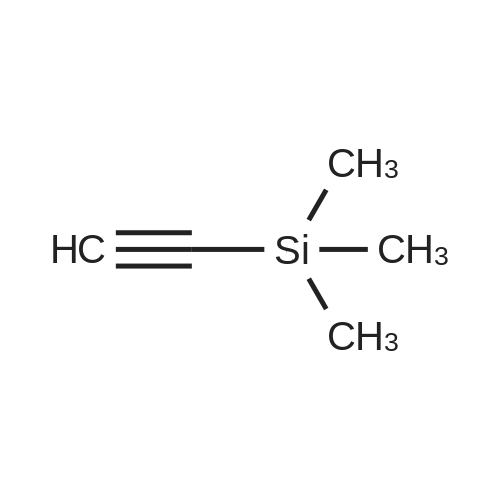
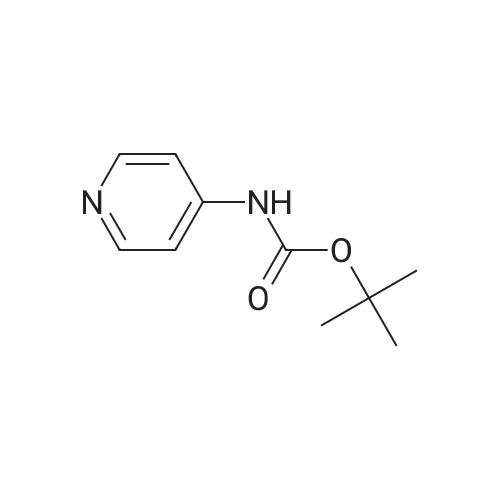
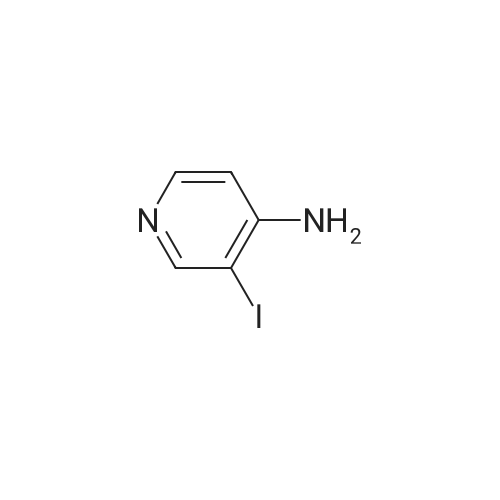
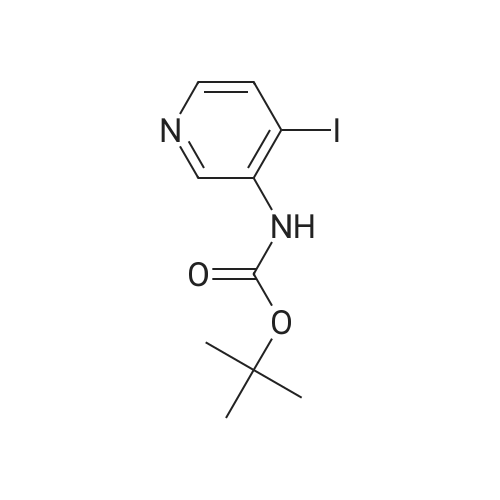
Propyne (6.3 mL, 124 mmol) was condensed in a pressure tube AT-78 C. A solution of (3- iodopyridin-4-yl) carbamic acid tert-butyl ester (7.98 g, 24.9 mmol) in 15 mL OF DMF, 60 mL of triethylamine, dichlorobis (triphenylphosphine) palladium (II) (877 mg, 1.25 mmol), and Cul (472 mg, 2.49 mmol) were added, and the tube was sealed and stirred at room temperature overnight. 200 mL of ethyl acetate and 100 mL of saturated aqueous ammonium chloride solution were added, the phases were separated and the aqueous layer was extracted with three 100 mL portions of ethyl acetate. The combined organic layers were dried over magnesium sulfate, filtered, and concentrated IN VACUO. Chromatography on SI02 (25% to 40% ethyl acetate in hexanes, gradient) afforded 5.65 g of (3-prop-1-ynylpyridin-4-yl) carbamic acid tert- butyl ester (98% yield). (3-Prop-l-ynylpyridin-4-yl) carbamic acid tert-butyl ester (5.65 g, 24.3 mmol) was treated with 30. 4 mL of 4 M HC1 in dioxane. The mixture was sonicated until the compound completely dissolved. After 15 hours, the mixture was concentrated in vacuo. The resulting brown solid (4.10 g) was dissolved in 65 ML of L-METHYL-2-PYRROLIDINONE (NMP) and treated with tert- BUOY (7.08 g, 63.2 mmol) at room temperature. After 18 hours, 300 ML of ethyl acetate and 300 mL of water were added. The phases were separated and the aqueous layer was extracted with five 100 mL portions of ethyl acetate. The combined organic layers were washed with two 20 mL portions of water, dried over magnesium sulfate, filtered, and concentrated in vacuo. Chromatography on SI02 (10% methanol in dichloromethane) yielded 2.85 g of the title product as yellow solid (89% yield). |
| 97% |
With triethylamine;bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; at -78 - 20℃; for 5 - 6h; |
Propyne (8 ml) was condensed into triethylamine (40 ml) at -78 C and then added to a mixture of (3-iodo-pyridin-4-yl) -carbamic acid tert-butyl ester (19.59 g, 0.06 mol), copper (I) iodide (0.81 g, 4.25 mmol) and palladium bistriphenylphosphine dichloride (2.09 g, 2.98 mmol) in triethylamine (25 ml) in a pressure tube at -60 C. The mixture was stirred at room temperature for 6 h and then diluted with ethyl acetate (1000 ml), washed with water (2 x 500 ml), dried and concentrated in vacuo to leave a residue. Purification by flash column chromatography on silica gel eluting with 40 % ethyl acetate: heptane gave (3-prop-l-ynyl-pyridin-4-yl)-carbamic acid tert-butyl ester (13.74 g, 97 %) as a yellow oil which solidified on standing, No.H (400 MHz, CDCI3) 8.51 (1H, s), 8.39 (1H, s), 8.07 (1H, s), 7.31 (1H, s), 2.18 (3H, s) 1.55 (9H, s) ; Tr = 1.00 min, m/z (ES+) (M+H)+ 233.09. |
|
With copper(l) iodide; triethylamine;bis-triphenylphosphine-palladium(II) chloride; In N,N-dimethyl-formamide; at -78 - 20℃; for 1h; |
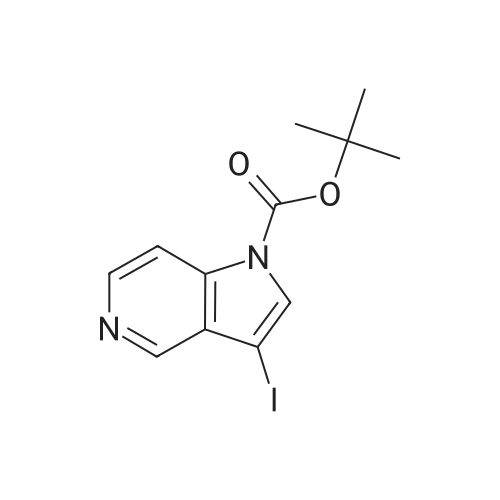
2-methyl- 1 H-pyrrolo [3,2-c] pyridine; [00383] Prop-l-yne (1.06 mL, 18.7 mmol) was condensed at -78 C, after which a reddish-brown solution of tert-butyl 3 -iodopyridin-4-yl carbamate (2.00 g, 6.25 mmol, Alfa Aesar), copper (I) iodide (0.1 19 g, 0.625 mmol), bis(triphenylphosphine)palladium (II) chloride (0.219 g, 0.312 mmol), and triethylamine (4.79 mL, 34.4 mmol) in N,N- dimethylformamide (5.3 mL) was added. The reaction became olive green upon addition at - 78 C, and was then warmed to room temperature and stirred at room temperature for one hour. The reaction was then shown to be complete by LCMS analysis, after which the reaction mixture was diluted in water, extracted with ethyl acetate (3 x 50 mL), dried (sodium sulfate), filtered and concentrated to a brown residue which was purified on silica gel (ISCO 80g, 20 mL/min) using 10 to 50% ethyl acetate in hexanes over 60 minutes. The product, tert-butyl 3 -(prop- 1 -ynyl)pyridin-4-yl carbamate (1.54 g, 6.63 mmol, 106 % yield) was isolated as a gold oil (trapped with -6% ethyl acetate by NMR). NMR (400 MHz, CDC13) delta (ppm): 8.47 (s, 1H), 8.35 (d, 1H), 8.05 (d, 1H), 7.31 (br. s, 1H), 2.18 (s, 3H), 1.55 (s, 9H).[00384] To a solution of tert-butyl 3-(prop-l-ynyl)pyridin-4-ylcarbamate (1.54 g, 6.63 mmol, with 6% residual ethyl acetate) in MeOH (14.7 mL) was added 1,8- diazabicyclo[5.4.0]undec-7-ene (3.00 mL, 19.9 mmol). The reaction was then stirred at 70 C for 60 hours, after which it was complete by LCMS analysis after which it was concentrated and purified directly on silica gel (Luknova 80g, 20 mL/min) using 3 to 10% methanol in dichloromethane over 45 minutes. The product, 2-methyl- lH-pyrrolo[3, 2- c]pyridine (729 mg, 5.52 mmol, 83 % yield) was isolated as a tan solid. NMR (400 MHz, CD3OD) delta (ppm): 8.60 (d, 1H), 8.03 (d, 1H), 7.29 (m, 1H), 6.30 (s, 1H), 2.45 (s, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping