|
With caesium carbonate; In DMF (N,N-dimethyl-formamide); at 140℃; for 2h; |
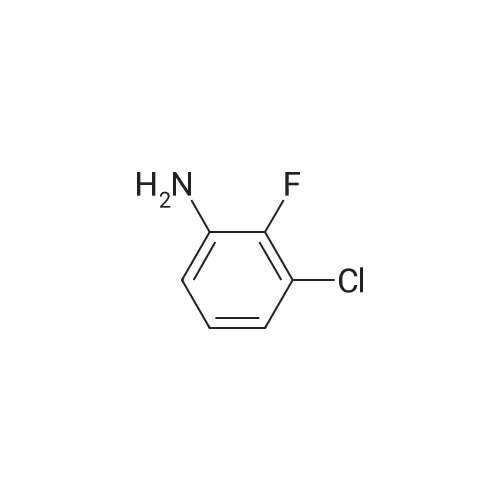
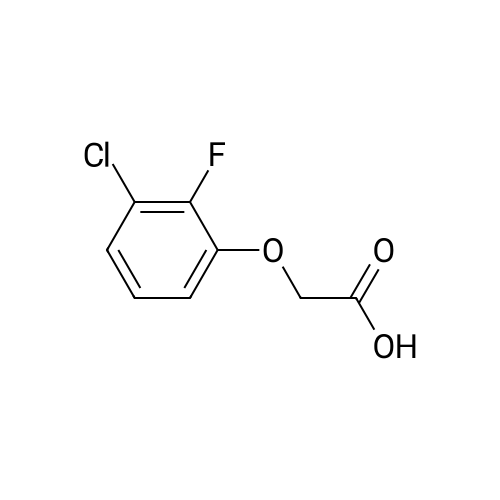
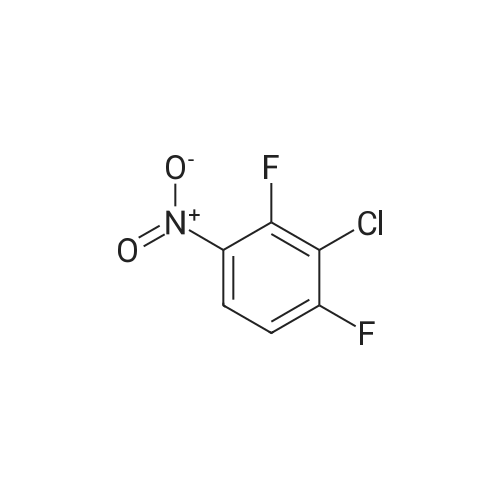
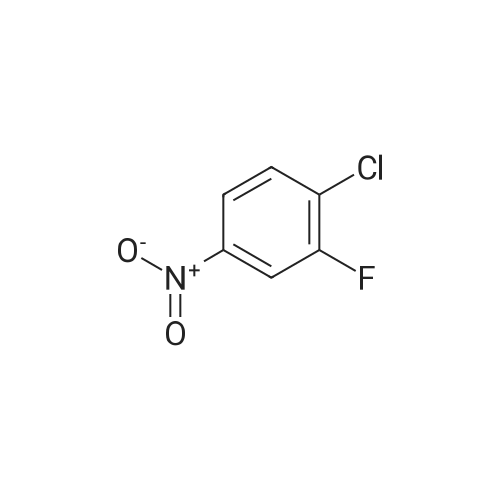
A mixture of a methyl 2-aminobenzoate (454 mg, 3.0 mmol), 3-chloro- 2-fluoronitrobenezene (352 mg, 2 mmol) and CS2CO3 (0.78 g, 2.4 mol) in DMF (4 mL) was stirred at 140C for 2 h. [0212] The mixture was diluted with EtOAc (10 mL) and washed with 2 M aqueous NaOH-solution (2 x 5 mL), dried (Na2S04), concentrated and flash chromatographed (Si02, toluene: heptane : EtOAc-system) and concentrated. The residue was taken up in THF (10 mL), 1 M aqueous LiOH (5 mL) was added and the resulting mixture was stirred at 80C for 1 h, and then allowed to obtain room temperature. 2 M aqueous HC1 was added until pH 2. The aqueous phase was extracted with EtOAc (3 x). The combined organic phases were dried (Na2S04) and concentrated. The residue was taken up in EtOH and a mixture of K2C03 (1.38 g, 10 mmol) and Na2S204 (1.74 g, 10 mmol) in water was added and the resulting mixture was stirred for 1 h. The mixture was diluted with water and washed with 1 M aqueous NaOH-solution (2 x 5 mL) and then dried (Na2S04) and concentrated. |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 140℃; for 2h; |
A mixture of a methyl 2-aminobenzoate (454 mg, 3.0 mmol), 3-chloro-2- fluoronitrobenezene (352 mg, 2 mmol) and Cs2CO3 (0.78 g, 2.4 mol) in DMF (4 mL) was stirred at 1400C for 2 h.[0214] The mixture was diluted with EtOAc (10 mL) and washed with 2 M aqueous NaOH-solution (2 x 5 mL), dried (Na2SO4), concentrated and flash chromatographed (SiO2, toluene:heptane:EtOAc-system) and concentrated. The residue was taken up in THF (10 mL), 1 M aqueous LiOH (5 mL) was added and the resulting mixture was stirred at 8O0C for 1 h, and then allowed to obtain room temperature. 2 M aqueous HCl was added until pH 2. The aqueous phase was extracted with EtOAc (3 x). The combined organic phases were dried (Na2SO,)) and concentrated. The residue was taken up in EtOH and a mixture OfK2CO3 (1.38 g, 10 mmol) and Na2S2O4 (1.74 g, 10 mmol) in water was added and the resulting mixture was stirred for 1 h. The mixture was diluted with water and washed with 1 M aqueous NaOH-solution (2 x 5 mL) and then dried (Na2SO4) and concentrated.[0215J The residue was taken up in CH2Cl2 and l-ethyl-3-(3- dimethylaminopropyl)carbodiimide hydrochloride (307 mg, 1.6 mmol) was added. The resulting mixture was stirred at room temperature for 1 h. The mixture was diluted with EtOAc, washed with saturated aqueous NaHCO3-solution, dried (Na2SO4), concentrated, and flash chromatographed (SiO2, heptane:EtOAc, 2: 1) to give 21 mg of the intermediate lactam.[0216] The intermediate lactam was taken up in dioxane and added to a mixture of TiCU (0.19 mL, 0.19 mmol, 1 M in toluene) and piperazine (73 mg, 0.85 mmol) in <n="75"/>dioxane at 500C. The resulting mixture was stirred at 1000C over night, and then allowed to obtain room temperature. To the mixture was added aqueous HCl (1 mL, 2 M) and then the aqueous phase was extracted with EtOAc (2 x 1 mL). To the aqueous phase was added aqueous NaOH (2 mL, 2 M) and the resulting suspension was extracted with EtOAc (3 x ImL). The combined organic phases were concentrated and purified by HPLC to give 9.8 mg of the title compound (189JO68) MS (ESI) 313 (MH+). Purity for MH+ (UV/MS) 100/98. |
|
With caesium carbonate; In N,N-dimethyl-formamide; at 140℃; for 2h; |
A mixture of a methyl 2-aminobenzoate (454 mg, 3.0 mmol), 3-chloro-2- fluoronitrobenezene (352 mg, 2 mmol) and Cs2CO3 (0.78 g, 2.4 mol) in DMF (4 mL) was stirred at 1400C for 2 h.[0249] The mixture was diluted with EtOAc (10 mL) and washed with 2 M aqueous NaOΗ-solution (2 x 5 mL), dried (Na2SO4), concentrated and flash chromatographed (SiO2, toluene:heptane:EtOAc-system) and concentrated. The residue was taken up in TΗF (10 mL), 1 M aqueous LiOH (5 mL) was added and the resulting mixture was stirred at 800C for 1 h, and then allowed to obtain room temperature. 2 M aqueous HCl was added until pΗ 2. The aqueous phase was extracted with EtOAc (3 x). The combined organic phases were dried (Na2SO4) and concentrated. The residue was taken up in EtOH and a mixture OfK2CO3 (1.38 g, 10 mmol) and Na2S2O4 (1.74 g, 10 mmol) in water was added and the resulting mixture was stirred for 1 h. The mixture was diluted with water and washed with 1 M aqueous NaOΗ-solution (2 x 5 mL) and then dried (Na2SO4) and concentrated.[0250] The residue was taken up in CH2Cl2 and l-ethyl-3-(3- dimethylaminopropyl)carbodiimide hydrochloride (307 mg, 1.6 mmol) was added. The EPO <DP n="91"/>resulting mixture was stirred at room temperature for 1 h. The mixture was diluted with EtOAc, washed with saturated aqueous NaHCO3-solution, dried (Na2SO4), concentrated, and flash chromatographed (SiO2, heptane: EtOAc, 2:1) to give 21 mg of the intermediate lactam. [0251] The intermediate lactam was taken up in dioxane and added to a mixture of TiCl4 (0.19 niL, 0.19 mmol, 1 M in toluene) and piperazine (73 mg, 0.85 mmol) in dioxane at 50C. The resulting mixture was stirred at 1000C over night, and then allowed to obtain room temperature. To the mixture was added aqueous HCl (1 mL, 2 M) and then the aqueous phase was extracted with EtOAc (2 x 1 mL). To the aqueous phase was added aqueous NaOH (2 mL, 2 M) and the resulting suspension was extracted with EtOAc (3 x ImL). The combined organic phases were concentrated and purified by HPLC to give 9.8 mg of the title compound (189JO68) MS (ESI) 313 (MH+). Purity for MH+ (UVMS) 100/98. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping