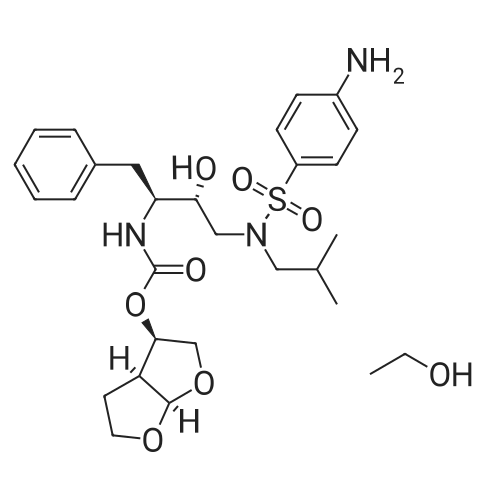
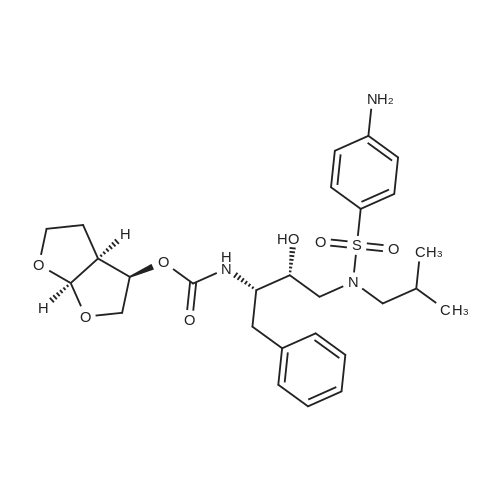
Oral and Inhaled Fosamprenavir Reverses Pepsin-Induced Damaged in a Laryngopharyngeal Reflux Mouse Model
Johnston, Nikki
;
Samuels, Tina L.
;
Goetz, Christopher J.
, et al.
Laryngoscope,2023,133(S1):S1-S11.
DOI:
10.1002/lary.30242
PubMed ID:
35678265
More
Abstract: Objective: More than 20% of the US population suffers from laryngopharyngeal reflux. Although dietary/lifestyle modifications and alginates provide benefit to some, there is no gold standard medical therapy. Increasing evidence suggests that pepsin is partly, if not wholly, responsible for damage and inflammation caused by laryngopharyngeal reflux. A treatment specifically targeting pepsin would be amenable to local, inhaled delivery, and could prove effective for endoscopic signs and symptoms associated with nonacid reflux. The aim herein was to identify small molecule inhibitors of pepsin and test their efficacy to prevent pepsin-mediated laryngeal damage in vivo.
Methods: Drug and pepsin binding and inhibition were screened by high-throughput assays and crystallography. A mouse model of laryngopharyngeal reflux (mechanical laryngeal injury once weekly for 2 weeks and pH 7 solvent/pepsin instillation 3 days/week for 4 weeks) was provided inhibitor by gavage or aerosol (fosamprenavir or darunavir; 5 days/week for 4 weeks; n = 3). Larynges were collected for histopathologic analysis.
Results: HIV protease inhibitors amprenavir, ritonavir, saquinavir, and darunavir bound and inhibited pepsin with IC50 in the low micromolar range. Gavage and aerosol fosamprenavir prevented pepsin-mediated laryngeal damage (i.e., reactive epithelia, increased intraepithelial inflammatory cells, and cell apoptosis). Darunavir gavage elicited mild reactivity and no discernable protection; aerosol protected against apoptosis.
Conclusions: Fosamprenavir and darunavir, FDA-approved therapies for HIV/AIDS, bind and inhibit pepsin, abrogating pepsin-mediated laryngeal damage in a laryngopharyngeal reflux mouse model. These drugs target a foreign virus, making them ideal to repurpose. Reformulation for local inhaled delivery could further improve outcomes and limit side effects.
Keywords:
Laryngopharyngeal reflux ;
LPR ;
pepsin ;
fosamprenavir ;
darunavir
Purchased from AmBeed:
206361-99-1
Investigations into Alcohol Functionalization via Barton-McCombie Type Deoxygenation
Chufan Andrew Jin
;
University of North Carolina,2023.
DOI:
10.17615/8bb9-3k97
More
Abstract: Reactive species that are employed as electrophiles in functionalization of substrates are typically prepared from native functionality such as alcohols and carboxylic acids. The addition of a pre-functionalization step of native substrates can reduce the efficiency of syntheses. These pre-functionalization reactions along with the functionalization reactions themselves proceed through polar mechanisms, limiting the scope of both functionalization and pre-functionalization reactions. Reported herein are preliminary efforts in the development of a deoxy-functionalization protocol for secondary and tertiary alcohols. The reported protocol proceeds through a derived Barton-McCombie deoxygenation mediated by a super silyl radical. Transfer of a thiocarbonate activating group to the silyl radical generates carbon radical which can be trapped out with an external sulfonyl trap, generating sulfonyl radical, which may then react with an allylic silane allowing for turnover of the radical chain.
Purchased from AmBeed:
206361-99-1 ;
60-12-8 ;
226700-81-8

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping