|
|
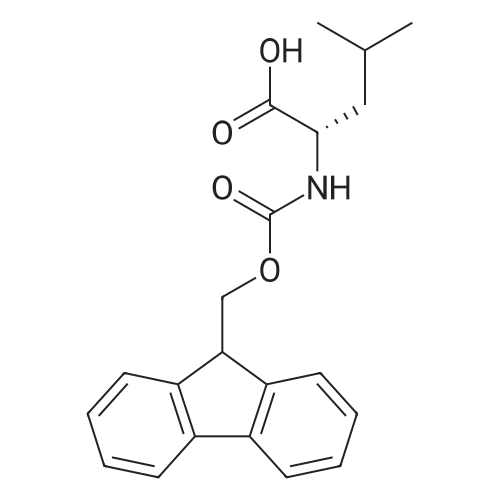
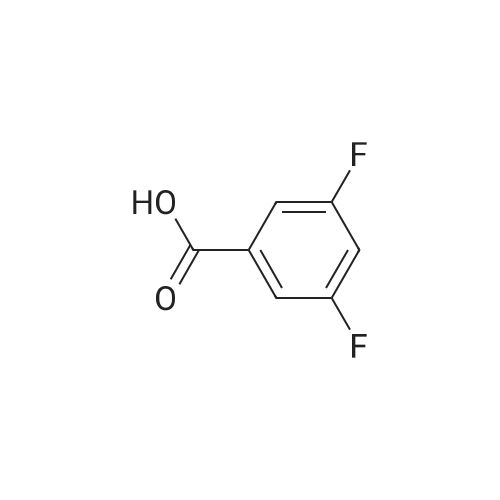
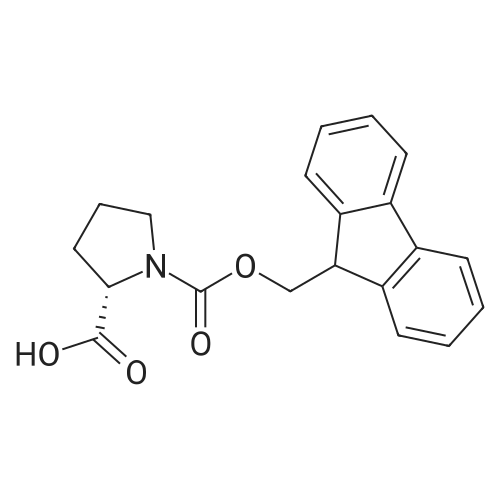
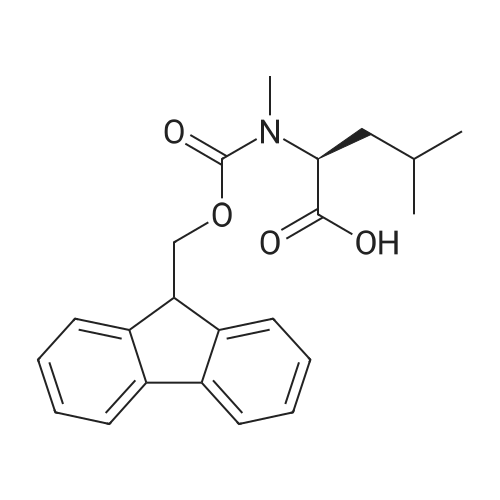
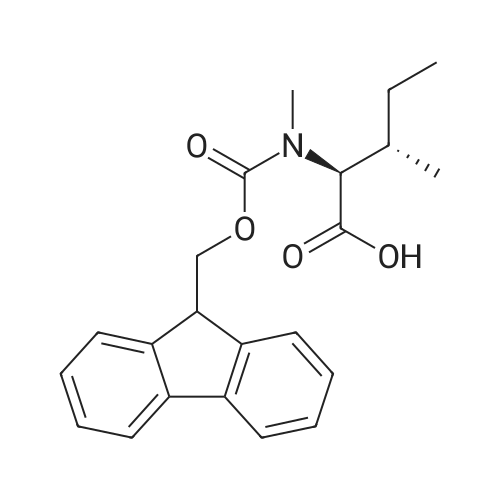
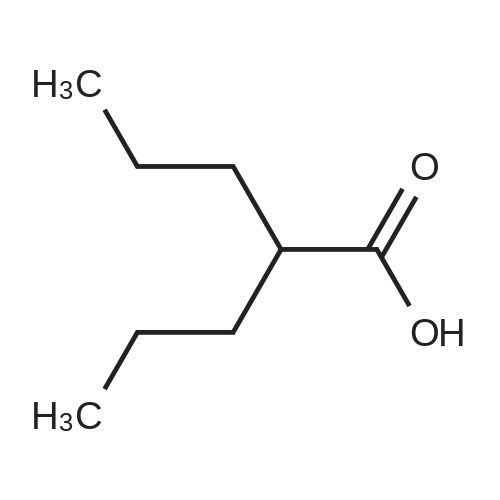
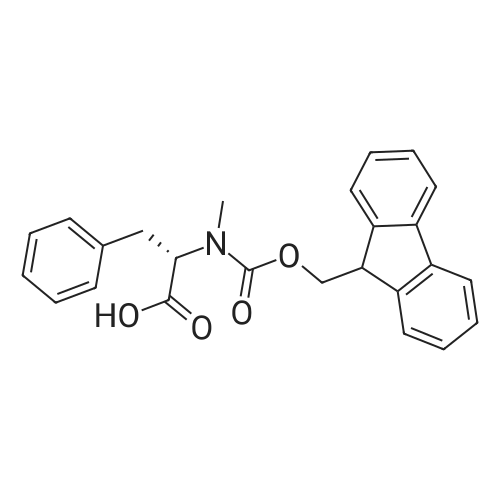
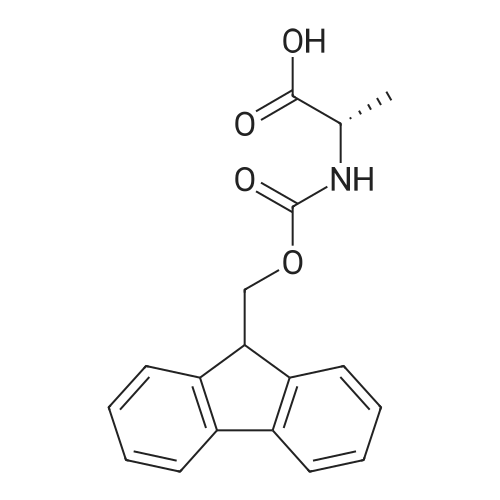
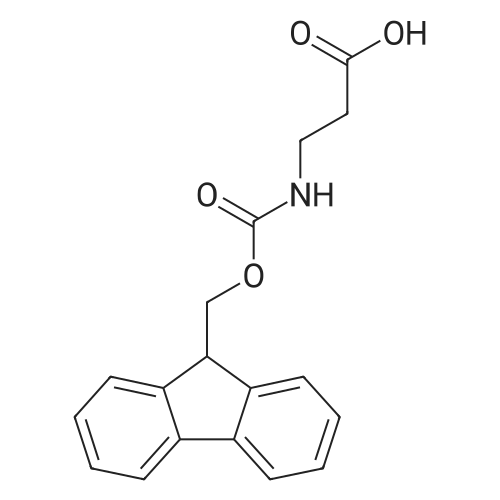
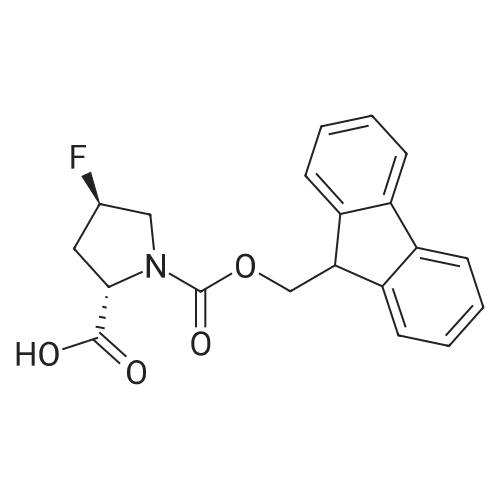
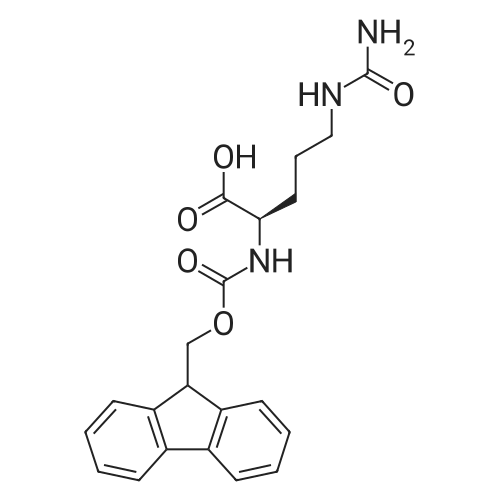
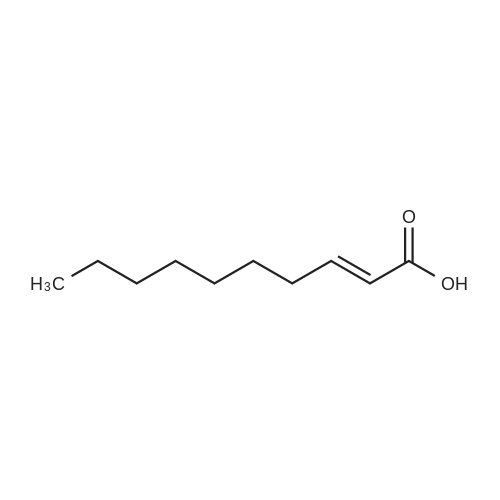
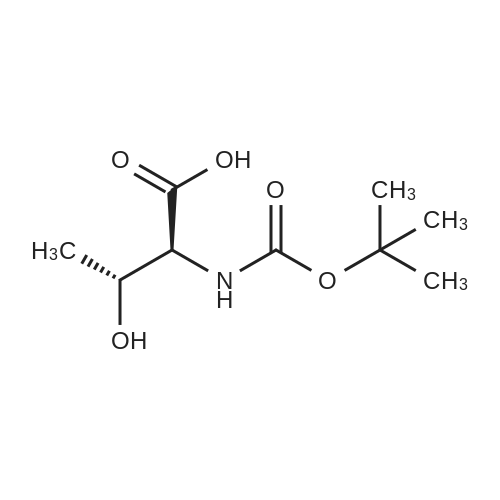
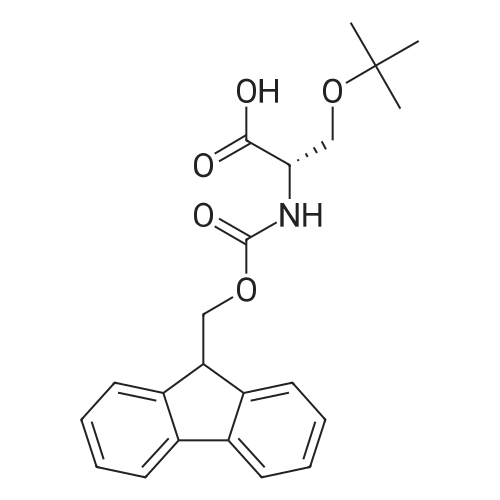
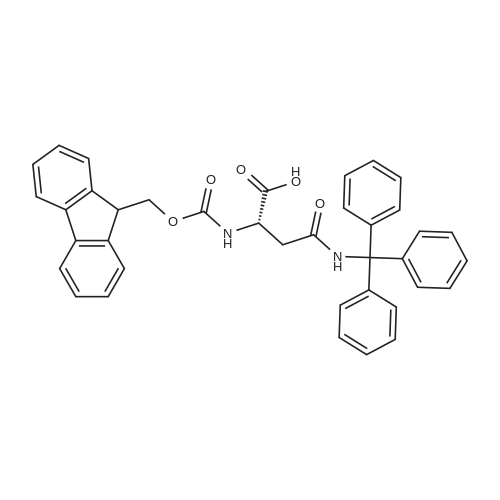
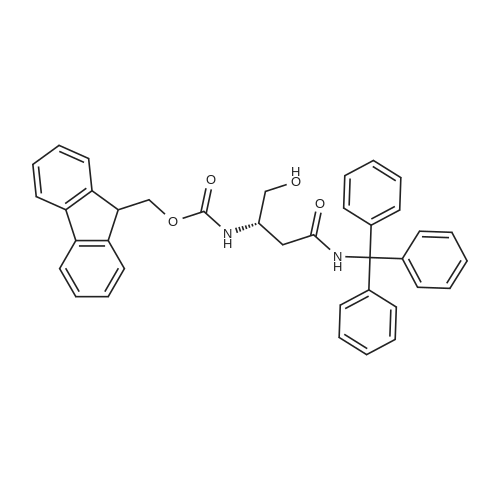
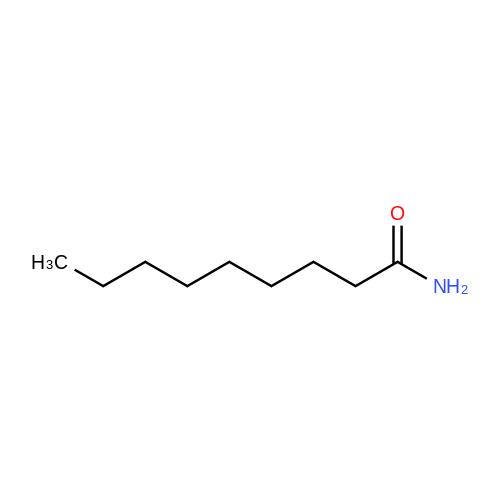
1.5 eq of commercially available Fmoc-NH-OH and DIEA (10 eq) are added to the 2-chlorotrityl resin in 2 mL DCM. The mixture is intermittently stirred manually during 24h. After that, 0.5 mL/g of MeOH are added to the reaction mixture to cap the remaining reactive points of the resin. After 15 minutes, the solution is filtered off and the resin is washed thoroughly with DCM, DMF and MeOH. Fmoc removal is achieved by treating the resin with 20% piperidine in DMF (1 x 5', 1 x 10' and 1 x 15'). For the coupling of Na-Fmoc-NY-alloc-L-2,4-diaminobutyric acid (Fmoc-L-Dab(alloc)-OH), 3 eq of the amino acid, 3 eq of the coupling agent DIC and 3 eq of oxyma pure dissolved in a small amount of DMF and premixed for 2 minutes. The resulting mixture is added to the resin and the reaction is allowed to proceed for 60 minutes. To extent of the reaction is monitored using the Kaiser test. The Fmoc group is then removed by treatments with 20% piperidine in DMF (1 x 5', 1 x 10' and 1 x 15 '). After that, Fmoc- N-methyl-L-iso leucine (Fmoc-NMe-L-Ile-OH) moiety is attached, for that purpose 3 eq of the amino acid, 3 eq of the coupling agent DIC and 3 eq of oxyma pure are dissolved in a small amount of DMF and premixed for 2 minutes. The resulting mixture is added to the resin and the reaction is allowed to proceed for 60 minutes. The extent of the reaction is monitored using the Kaiser test. The Fmoc group is then removed by treatments with 20% piperidine in DMF (1 x 5', 1 x 10' and 1 x 15 '). After that, Fmoc- L-Proline (Fmoc-L-Pro-OH) moiety is attached, for that purpose 3 eq of the amino acid, 3 eq of the coupling agent DIC and 3 eq of oxyma pure are dissolved in a small amount of DMF and premixed for 2 minutes. The resulting the mixture is added to the resin and the reaction is allowed to proceed for 60 minutes. The extent of the reaction is monitored using the Kaiser test. The Fmoc group is then removed by treatments with 20% piperidine in DMF (1 x 5', 1 x 10' and 1 x 15') and additional treatment with a mixture of piperidine/DBU/toluene/DMF (5:5:20:70) (1 x 5'). After that, Fmoc-N- methyl-L-leucine (Fmoc-NMeLeu-OH) moiety is attached, for that purpose 3 eq of the amino acid, 3 eq of the coupling agent DIC and 3 eq of oxyma pure are dissolved in a small amount of DMF and premixed for 2 minutes. The resulting mixture is added to the resin and the reaction is allowed to proceed for 60 minutes. The extent of the reaction is monitored using the Kaiser test. The Fmoc group is then removed by treatments with 20% piperidine in DMF (1 x 5', 1 x 10' and 1 x 15'). After that, Na- Fmoc-N(in)-Boc-N-methyl-L-tryptophan (Fmoc-NMe-L-Trp(Boc)-OH) moiety is attached, for that purpose 3 eq of the amino acid, 3 eq of the coupling agent DIC and 3 eq of oxyma pure are dissolved in a small amount of DMF and premixed for 2 minutes. The resulting mixture is added to the resin and the reaction is allowed to proceed for 60 minutes. The extent of the reaction is monitored using the Kaiser test. The Fmoc group is then removed by treatments with 20% piperidine in DMF (1 x 5', 1 x 10' and 1 x 15'). Butiric acid is coupled to the tryptophan moiety by adding to the resin 3 eq of the acid, 3 eq of the coupling agent DIC and 3 eq of oxyma pure are dissolved in a small amount of DMF and premixed for 2 minutes. The resulting the mixture is added to the resin and the reaction is allowed to proceed for 60 minutes. Then the reaction is filtered off and the resin is rinsed thoroughly with DMF and DCM. The extent of the reaction is monitored using the chloranil test. For the removal of the Alloc group, 10 eq of phenylsilane in DCM are added to the resin while N2 is bubbled through the mixture. Then, 0.1 eq of Pd(PPli3)4 are added maintaining the N2 bubbling while mixing everything well. Then the reaction vessel is sealed and shaken for 15 minutes. After this time, the reaction is filtered and the resin washed thoroughly. The same treatment is repeated two more times. After the last treatment, the resin is washed thoroughly with DCM, MeOH and DMF. For the coupling of the 3,5-difluorobenzoic acid on the side chain of the diaminoethyl moiety, 3 eq of said acid, 3 eq of the coupling agent DIC and 3 eq of oxyma pure dissolved in a small amount of DMF and premixed for 2 minutes. The resulting mixture is added to the resin and the reaction is allowed to proceed for 60 minutes. After this time, the resin is washed with DMF and DCM and the extent of the reaction is monitored the Kaiser test. For the cleavage of the peptide, the resin is washed several times with DCM and dried by suction. The peptide is cleaved from the resin by adding a solution of DCM/TFA (95:5), the mixture is allowed to react for 15 min. Then the reaction mixture is filtered and the resin rinsed with DCM. This cleavage procedure is repeated twice. All the filtrates are pooled and the solvent is evaporated under vacuum, yielding example 14. The compound is purified using reverse-phase chromatography. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping