| 94% |
With water; lithium hydroxide; In tetrahydrofuran; at 0 - 20℃; for 2h; |
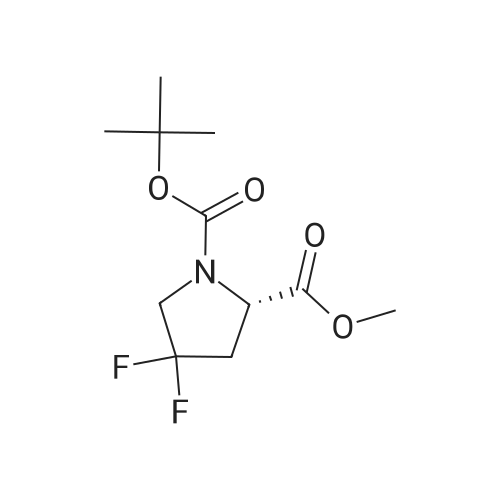
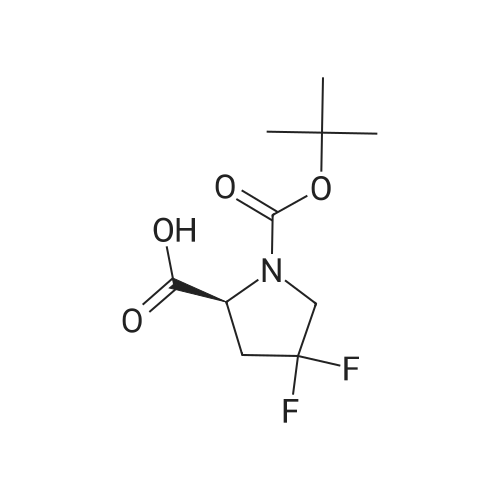
To a solution of compound 12-7 (5.0 g.18.86 mmol) in THF (40.0 mL) was added LiOH aqueous solution (1.5 g, 20 mL) at 0 "C, and the mixture was stirred at rt for 2.0 hrs. After the reaction was completed, the mixture was adjusted to pH 5 with diluted hydrochloric acid (1 M). and the solvent THF was removed in vacuo. The aqueous layer was adjusted to pH 2 with diluted hydrochloric acid (1 M). The resulting mixture was extracted with EtOAc (80 mL x 3). The combined organic layers were dried over anhydrous Na2S04and concentrated in vacuo to give the title compound as a white solid (4.45 g, 94%). The compound was characterized by the following spectroscopic data:MS (ESI. pos.ion) mlz: 252.23 [M+H]"; and NMR (400 MHz. CDCh) S (ppm): 9.60 (brs.1H).4.94-4.72.4.60-4.57 (m. m. 1H).3.89-3.74 (m.2H). 2.78-2.48 (m.2H).1.44 (d.9H../= 16 Hz). |
| 94% |
With lithium hydroxide; In tetrahydrofuran; water; at 0 - 20℃; for 2h; |
To a solution of compound 5-10 (5.0 g, 18.86 mmol) in THF (40 mL) at 0 C was added an aqueous solution of lithium hydroxide (1.5 g, 20 mL) dropwise. At the end of the addition, the mixture was stirred at rt for 2 hours. After the reaction was completed, the mixture was acidified with aqueous HC1 (1 M) till pH = 5 and then the THF was removed in vacuo. The aqueous layers were acidified with aqueous HC1 (1 M) till pH = 2 and extracted with EtOAc (80 mL x 3). The combined organic layers were washed with a saturated aqueous solution of NaCl, dried over anhydrous Na2SC>4 and concentrated in vacuo to give the title compound as a white solid (4.54 g, 94%). The compound was characterized by the following spectroscopic data: MS (ESI, pos.ion) mlz: 252.3 [M+H]+; and NMR (400 MHz, CDC13): delta 9.60 (brs, IH), 4.60-4.57, 4.94-4.72 (m, m, IH), 3.89-3.74 (m, 2H), 2.78-2.48 (m, 2H), 1.44 (d, 9H, J= 16 Hz) ppm. |
| 94% |
With water; lithium hydroxide; In tetrahydrofuran; at 0 - 20℃; for 2h; |
11125] To a solution of compound 14-3 (5.0 g, 18.86 mmol) in THF (40 mE) at 0 C. was added EiOH aqueous solution (1.5 g, 20 mE), and the mixture was stirred at it for 2 hrs. After the reaction was completed, the mixture was adjusted to pH 5 with diluted hydrochloric acid (1 M), and the solvent THF was removed in vacuo. The aqueous layer was adjusted to pH 2 with diluted hydrochloric acid (1 M). The resulting mixture was extracted with EtOAc (80 mEx3). The combined organic layers were dried over anhydrous Na2504 and concentrated in vacuo to give the title compound as a white solid (4.54 g, 94%). The compound was characterized by the following spectroscopic data:11126] MS (ESI, pos.ion) mlz: 252.23 [M+H]11127] ?H NMR (400 MHz, CDC13) oe (ppm): 9.60 (brs, 1H), 4.94-4.72, 4.60-4.57 (m, m, 1H), 3.89-3.74 (m, 2H),2.78-2.48 (m, 2H), 1.44 (d, 9H, J=16 Hz). |
| 94% |
With water; lithium hydroxide; In tetrahydrofuran; at 0 - 20℃; for 2h; |
Compound 26-4 (5.0 g, 18.86 mmol) was dissolved in THF (40 mL) and an aqueouslithium hydroxide solution (1.5 g, 20 mL) was added to the system at 0 C. The mixturewas reacted at room temperature for 2.0 hours. After the reaction was completed, thereaction mixture was adjusted to pH 5 with dilute hydrochloric acid (1 M) to remove theTHF. The aqueous layer was adjusted to pH 2 with diluted hydrochloric acid (1 M) andextracted with EtOAc (80 mL x 3). The organic phases were combined, Wash with saturatedbrine, anhydrous Na 2SO 4Drying and concentration gave 4.54 g of white solid, yield: 94%. |
| 86% |
With water; potassium hydroxide; |
<strong>[203866-17-5](S)-1-tert-butyl 2-methyl 4,4-difluoropyrrolidine-1,2-dicarboxylate</strong> (1.51 g, 5.69 mmol), obtained from step 2, was dissolved in 6 mL of 1M potassium hydroxide solution. The solution was stirred overnight. The mixture was washed with ether, acidified, extracted with ethyl acetate, washed with brine, dried over sodium sulfate, filtered and evaporated to yield slightly brownish crystals. The crude mixture was used without further purification. Yield: 1.23 g, 86% 1H NMR (400 MHz, DMSO-d6): delta 1.38 (s, 9H), 2.32-2.48 (m, 1H), 2.78-2.98 (m, 1H), 3.65-3.77 (m, 2H), 4.31-4.37 (m, 1H), 13.0 (br s, 1H). MS (ESI) m/z 250.8 [M-H]- LC-MS (I) Rt 1.51 min, m/z 252.5 [M+H]+ (90%). |
|
With lithium hydroxide; In tetrahydrofuran; methanol; water; for 2h; |
Example 3C 4, 4-Difluoro-pyrrolidine-1, 2S-DICARBOXYLIC acid 1-tert-butyl ester The compound from Example 3B (1.80 g, 6. 78 mmol) was dissolved in 3 mL each OF MEOH and THF, then 6.8 mL of 1.7 N LIOH was added. After stirring for 2 hours, the mixture was concentrated in vacuo, and ethyl acetate and IN HCl were added. The organic extracts were dried with NA2SO4 and concentrated to provide the crude acid (1.82 g). |
|
|
40 mL of a 2 mol/L sodium hydroxide aqueous solution was added dropwise over a period of 4 minutes under ice cooling to a 152 mL methanol solution of 15.2 g of the compound obtained in step 3-2, after which the reaction mixture was stirred for 2.5 hours at the same temperature. Upon completion of the reaction, the methanol was distilled off under reduced pressure, 100 mL of chloroform was added to the aqueous layer thus obtained, and then 90 mL of 1 mol/L hydrochloric acid was added dropwise over a period of 6 minutes under ice cooling. Once it was confirmed that the aqueous layer was acidic, liquid separation was performed, and the aqueous layer was extracted with chloroform (30 mL × 2). The combined organic layer was washed with 50 mL of saturated brine and dried with magnesium sulfate, then the drying agent was filtered off and the solvent was distilled off under reduced pressure to obtain 14.1 g of residue (white solid). The residue thus obtained was crystallized from diisopropyl ether/n-hexane to obtain 12.6 g of the titled compound (colorless crystals). MS (ESI neg.) m/z : 250([M-H]-) 1H-NMR (300 MHz, DMSO-d6) delta (ppm) ; 1.36 & 1.41 (each-s, 9 H), 2.31 - 2.53 (m, 1 H), 2.69 - 3.02 (m, 1 H), 3.59 - 3.86 (m, 2 H) 4.30 - 4.43 (m, 1 H), 12.98 (brs, I H) |
|
With sodium hydroxide; water; In methanol; at 20℃; for 1.5h; |
The crude product of 1-tert-butyl 2-methyl (2S) -4, 4-difluoro-1, 2-pyrrolidinedicarboxylate obtained in Example 8-2 (3.9g) was dissolved in methanol (LLML) and then 1N NaOH (22mL) was added at room temperature. After stirring for 1.5hrs, the resulting mixture was washed with diethyl ether, acidified with 1N HC1 (30ML), and then was extracted with ethyl acetate. The combined organic layer was washed with saturated aqueous NACL, dried over MGS04, and concentrated in vacuo. The residue was triturated with hexane to give the target compound as a white powder (3. 1G). 1H-NMR (IN CDC13) : D 1. 62-1.30 (9H, m), 2.92-2. 39 (2H, M), 4.02-3. 60 (2H, m), 4.69-4. 41 (1H, m), 7.50 (1H, br-s). MS (ESI-) : m/z 250.19 (M-H). |
|
|
EXAMPLE 3C 4,4-Difluoro-pyrrolidine-1,2S-dicarboxylic acid 1-tert-butyl ester The compound from Example 3B (1.80 g, 6.78 mmol) was dissolved in 3 mL each of MeOH and THF, then 6.8 mL of 1.7 N LiOH was added. After stirring for 2 hours, the mixture was concentrated in vacuo, and ethyl acetate and 1N HCl were added. The organic extracts were dried with Na2SO4 and concentrated to provide the crude acid (1.82 g). |
|
With potassium hydroxide; |
Step 3: (S)-1-(iert-butoxycarbonyl)-4,4-difluoropyrrolidine-2-carboxylic acid (S)-1 -ieri-butyl 2-methyl 4,4-difluoropyrrolidine-1 ,2-dicarboxylate (1 .51 g, 5.69 mmol), obtained from step 2, was dissolved in 6 m l_ of 1 M potassium hydroxide solution. The solution was stirred overnight. The mixture was washed with ether, acidified, extracted with ethyl acetate, washed with brine, dried over sodium sulfate, filtered and evaporated to yield slightly brownish crystals. The crude mixture was used without further purification. Yield: 1 ,23g, 86% 1 H NMR (400 MHz, DMSO-c) : delta 1 .38 (s, 9H), 2.32 - 2,48 (m , 1 H), 2.78-2.98 (m, 1 H), 3.65 - 3.77 (m, 2H), 4.31 -4.37 (m , 1 H), 13.0 (br s, 1 H). MS (ESI) m/z 250.8 [M-H]~ LC-MS(I) Rt 1 .51 min, m/z 252.5 [M+H]+ (90%). |
|
With lithium hydroxide monohydrate; In tetrahydrofuran; methanol; water; at 20℃; for 1h; |
N-Boc-4,4-Difluoro-L-proline methyl ester (1.0 g, 3.77 mmol, 1.0 eq.) was dissolved in THF/MeOH (2 mL / 2 mL) and treated with a solution of lithium hydroxide monohydrate (316 mg, 7.53 mmol, 2.0 eq.) in water (4 mL). The resulting mixture was stirred at room temperature for lh, cooled to 0C, acidified with IN HCl solution, and then extracted three times with EtOAc. The combined organic phases were dried over Na2S04, filtered and concentrated under vacuum to give the desired product, which was used in the next step without further purification. |
|
|
N-Boc-4,4-Difluoro-L-proline methyl ester (1.0 g, 3.77 mmol, 1.0 eq.) was dissolved in THF/MeOH (2 mL/2 mL) and treated with a solution of lithium hydroxide monohydrate (316 mg, 7.53 mmol, 2.0 eq.) in water (4 mL). The resulting mixture was stirred at room temperature for 1 h, cooled to 0 C., acidified with 1N HCl solution, and then extracted three times with EtOAc. The combined organic phases were dried over Na2SO4, filtered and concentrated under vacuum to give the desired product, which was used in the next step without further purification. |
| 9 g |
With water; sodium hydroxide; In methanol; at 25℃; for 2h; |
To a solution of the product from the previous step (11 g, 41.47 mmol, 1.0 eq) in a mixture of THF (80 mL) and MeOH (80 mL) was added dropwise aqueous NaOH (2 M, 41.47 mL, 2.0 eq). The resulting mixture was stirred at 25 C. for 2 hr then concentrated under reduced pressure and partitioned between H2O (40 mL) and EtOAc (30 mL). The layers were separated; the aqueous phase was adjusted to pH 6 by addition of 2M aq. HCl, then extracted with EtOAc (50 mL×5). The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure to give the title compound (9 g) as a viscous oil. 1H NMR (400 MHz, CDCl3): delta 6.15 (br, 1H), 4.55-4.53 (m, 1H), 3.86-3.73 (m, 2H), 2.72-2.65 (m, 2H), 1.48 (s, 9H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping