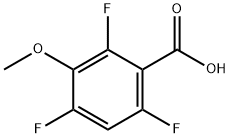
3-METHOXY-2,4,6-TRIFLUOROBENZOIC ACID
|
- $70 - $406
- Product name: 3-METHOXY-2,4,6-TRIFLUOROBENZOIC ACID
- CAS: 886499-94-1
- MF: C8H5F3O3
- MW: 206.12
- EINECS:
- MDL Number:MFCD06660225
- Synonyms:3-METHOXY-2,4,6-TRIFLUOROBENZOIC ACID;2,4,6-Trifluoro-3-Methoxybenzoic acid, 97%;Benzoic acid, 2,4,6-trifluoro-3-methoxy-
5 prices
Selected condition:
Brand
- Apolloscientific
- Crysdot
- SynQuest Laboratories
Package
- 1g
- 5g
- ManufacturerApolloscientific
- Product numberPC302097
- Product description3-Methoxy-2,4,6-trifluorobenzoicacid 97%
- Packaging1g
- Price$70
- Updated2021-12-16
- Buy
- ManufacturerApolloscientific
- Product numberPC302097
- Product description3-Methoxy-2,4,6-trifluorobenzoicacid 97%
- Packaging5g
- Price$270
- Updated2021-12-16
- Buy
- ManufacturerCrysdot
- Product numberCD12016672
- Product description3-Methoxy-2,4,6-trifluorobenzoicacid 95+%
- Packaging5g
- Price$406
- Updated2021-12-16
- Buy
- ManufacturerSynQuest Laboratories
- Product number2621-3-75
- Product description3-Methoxy-2,4,6-trifluorobenzoic acid
- Packaging1g
- Price$96
- Updated2021-12-16
- Buy
- ManufacturerSynQuest Laboratories
- Product number2621-3-75
- Product description3-Methoxy-2,4,6-trifluorobenzoic acid
- Packaging5G
- Price$298
- Updated2021-12-16
- Buy
| Manufacturer | Product number | Product description | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|
| Apolloscientific | PC302097 | 3-Methoxy-2,4,6-trifluorobenzoicacid 97% | 1g | $70 | 2021-12-16 | Buy |
| Apolloscientific | PC302097 | 3-Methoxy-2,4,6-trifluorobenzoicacid 97% | 5g | $270 | 2021-12-16 | Buy |
| Crysdot | CD12016672 | 3-Methoxy-2,4,6-trifluorobenzoicacid 95+% | 5g | $406 | 2021-12-16 | Buy |
| SynQuest Laboratories | 2621-3-75 | 3-Methoxy-2,4,6-trifluorobenzoic acid | 1g | $96 | 2021-12-16 | Buy |
| SynQuest Laboratories | 2621-3-75 | 3-Methoxy-2,4,6-trifluorobenzoic acid | 5G | $298 | 2021-12-16 | Buy |
Properties
Melting point :100-102℃
Boiling point :291.0±35.0 °C(Predicted)
Density :1.487±0.06 g/cm3(Predicted)
form :Solid
pka :2.16±0.10(Predicted)
Boiling point :291.0±35.0 °C(Predicted)
Density :1.487±0.06 g/cm3(Predicted)
form :Solid
pka :2.16±0.10(Predicted)
Safety Information
| Symbol(GHS): |

|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Signal word: | Warning | ||||||||||||||||||||||||||||
| Hazard statements: |
|
||||||||||||||||||||||||||||
| Precautionary statements: |
|
Description
More related product prices
2,4,6-Trifluorobenzoic acidRelated product price
- 2,4,6-Trifluorobenzoic acid
$6-2472.99





