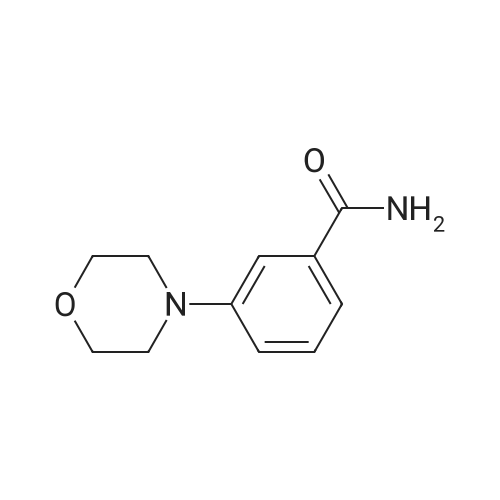
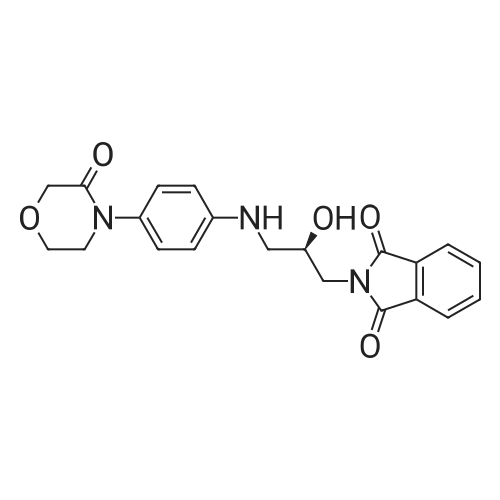
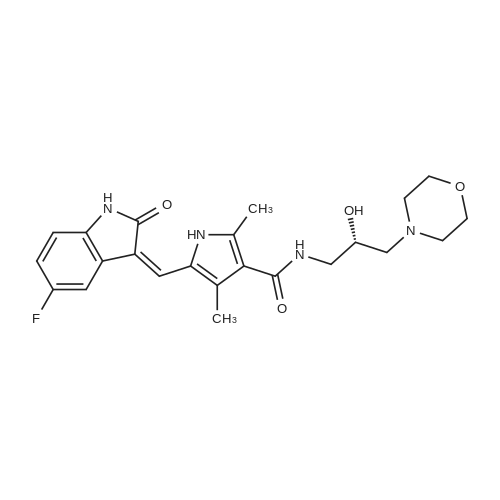
| 84% |
|
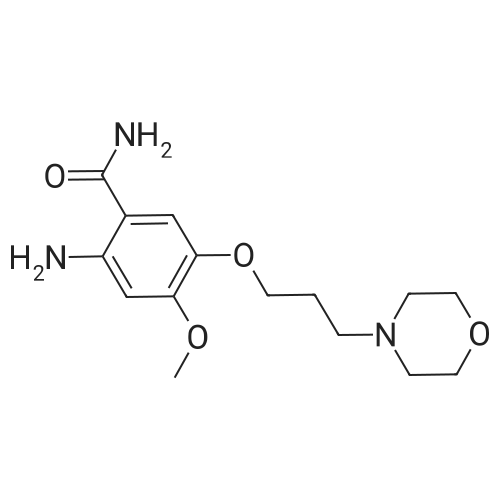
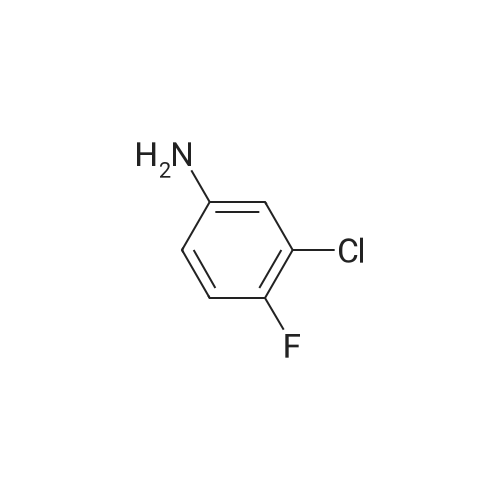
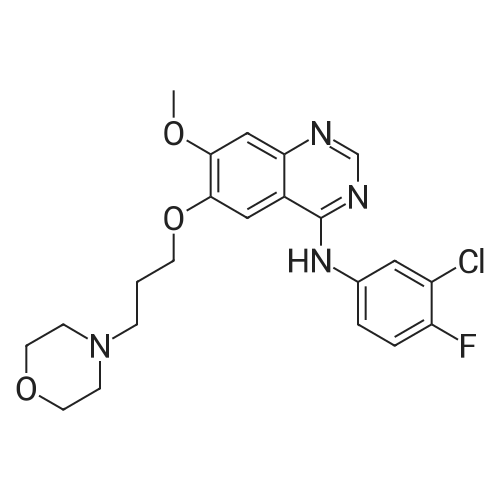
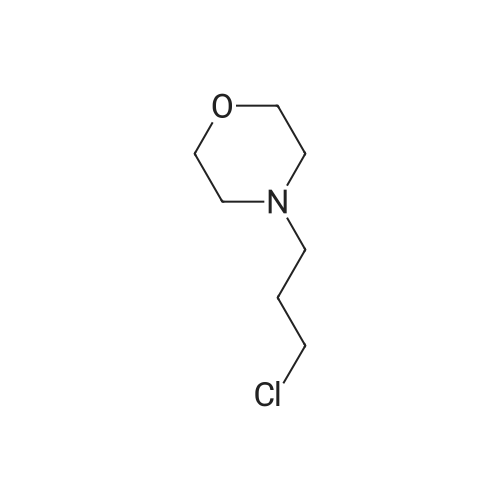
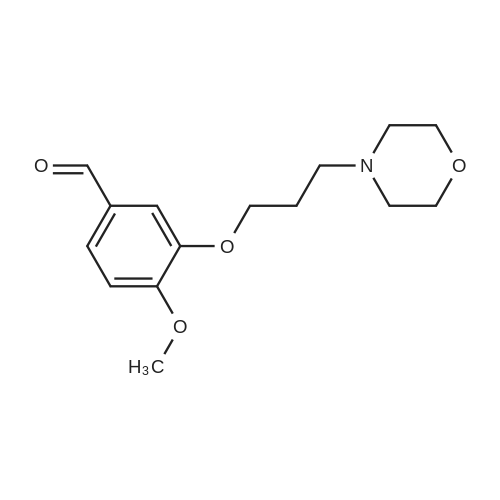
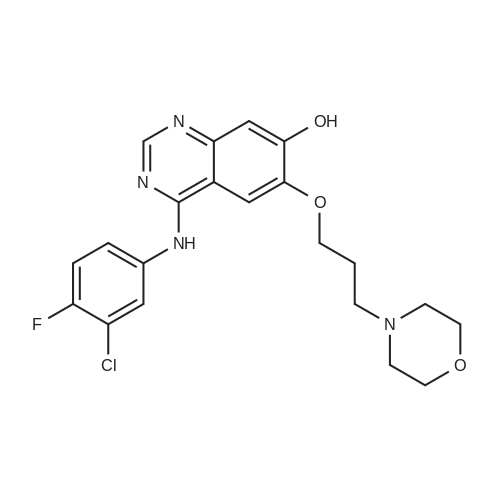
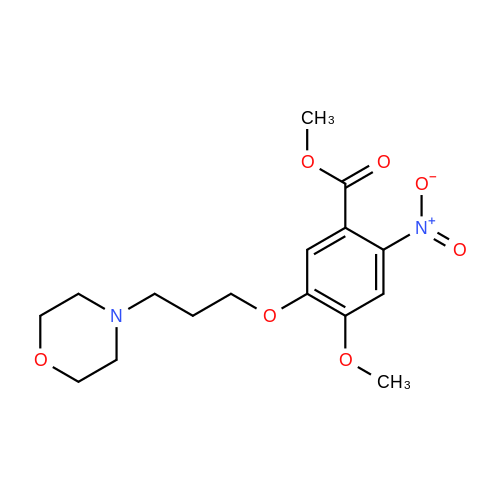
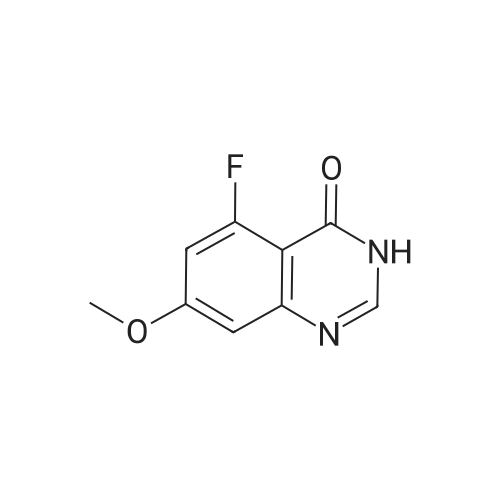
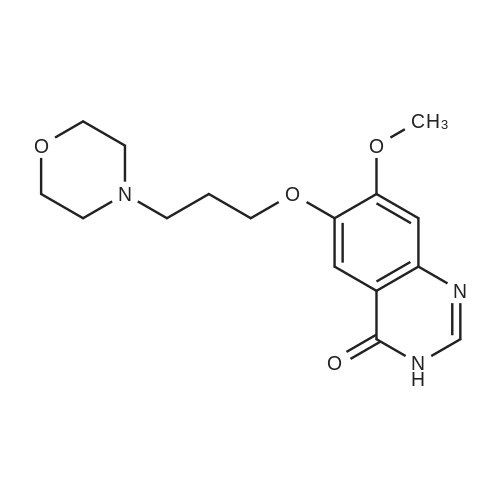
7 - methoxy -6 - [3 - (4 - morpholinyl) propoxy] quinazoline -4 (3H) - ketone (V) (7.5g, 23mmol), thionyl chloride (105 ml) and DMF (1.5g) after mixing the heating to reflux 1 hour, evaporate the solvent. The residue is added toluene (35 ml), concentrated under reduced pressure, repeated 3 times. The residue by adding isopropanol (35 ml), stirring at room temperature for 1 hour, filtered, filters cake Canada to 3 - chloro -4 - fluoro aniline (7.5g, 52mmol) isopropyl alcohol (80 ml) solution, stirring under heating to reflux 1 hour. Cooling to 30 °C, filtering, drying filter cake. The resulting solid re-dissolved in water (100 ml) and heating to the 60 °C PH add saturated sodium hydroxide solution adjusted to 9.5 - 10.0, after cooling crystallization, filtration, the filter cake is recrystallized with ethyl acetate, to obtain 4 - (3 - chloro -4 - fluoro benzyl amidogen) -7 - methoxy -6 - (3 - morpholino-propoxy) quinazoline (I) (8.8g), yield 84percent. |
| 82% |
With potassium carbonate; In isopropyl alcohol; at 80 - 85℃; for 1h; |
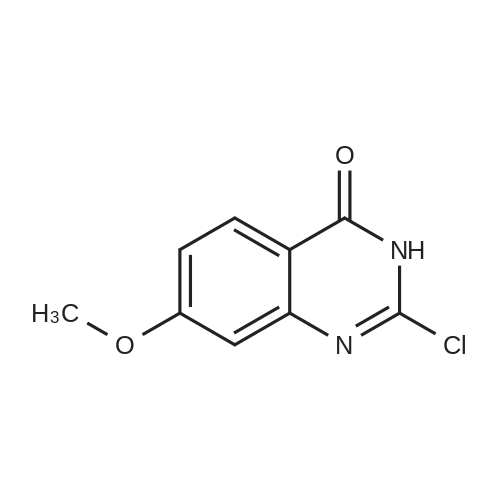
To the reaction tank, 121.3 g of 3-chloro-4-fluoroaniline and 1.8 L of isopropanol were added to the whole solution,219.6 g of potassium carbonate was added, 268.2 g of intermediate 1 was added, and the mixture was heated to 80 to 85 ° C for 1 hour.TLC monitoring (MeOH: DCM = 1: 5, ZJ1 Rf = 0.6, product Rf = 0.8), the feed point disappeared.Hot filter, the filtrate cooled to room temperature, get solid, filter, filter cake with leaching of isopropyl alcohol 1.5L after drying,Dried at 50-60 ° C for 6 h under reduced pressure to give crude gefitinib, pale yellow solid 333.5 g, yield: 94percent, HPLC purity 99.3percent Purification of crude gefitinibTo the reaction tank was added gefitinib crude 333.5 g, methyl isobutyl ketone 2L and ethyl acetate 6L, stir, literWarm reflux 3 hours, dissolved, hot filter, the filtrate naturally cooled to 20 ~ 25 ,The crystallization was carried out for 24 hours. The filter cake was dried at a temperature of 88 to 93 ° C under reduced pressure (-0.08 MPa) for 6 hours to obtain 273.5 g of white crystalline powder, which was gefitinib. mp: 193-195 ° C, yield: 82percent. HPLC showed that the single product was less than 0.1percent. |
| 62.86% |
|
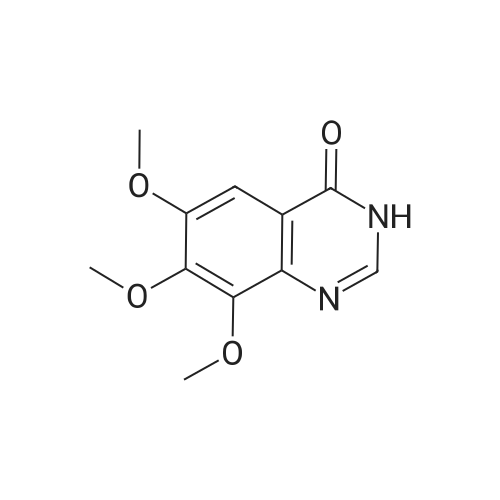
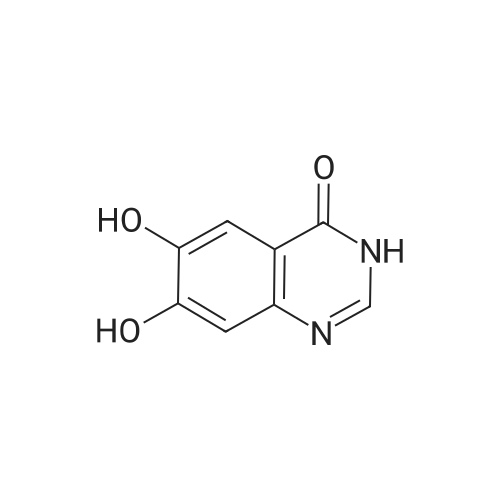
(XII) One-Pot Reaction; (1) Preparation of Gefitinib <strong>[199327-61-2]7-methoxy-6-(3-morpholinopropoxy)-3,4-dihydroquinazolin-4-one</strong> (29.12 g, 0.0913 mol) is dissolved in toluene, Et3N (19 ml, 0.1366 mol) is added at 5° C., and after POCl3 (17.8 ml, 0.1824 mol) is added, reaction is carried out for 3 hours at 70° C. 3-chloro-4-fluoroaniline (15.9 g, 0.1093 mol) mixed into the isopropyl alcohol (10 ml) is added to the above reaction solution, and then stirring is carried out for 1 hour at 70° C. Wheat solid compound is obtained by filter, water (380 ml) is added to dissolve the solid compound entirely, NaOH (30 ml, 20percent) is added, and after stirred for 1 hour, filter is carried out. After dissolved solid and filtered, Gefitinib (25.65 g, 62.86percent) that is white solid compound is obtained, whose purity determined by HPLC is greater than 99.9percent. 1H-NMR (DMSO) spectrum: 2.21 (brs, 2H), 2.84 (brs, 4H), 2.92 (brs, 2H), 3.80 (brs, 4H), 3.99 (s, 3H), 4.28 (brs, 2H), 7.15 (s, 1H), 7.24 (t, 1H, J=8.9 Hz), 7.71 (m, 2H) 8.00 (m, 1H), 8.44 (s, 1H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping