|
With diisobutylaluminium hydride; triethylamine; In hexane; dichloromethane; water; dimethyl sulfoxide; |
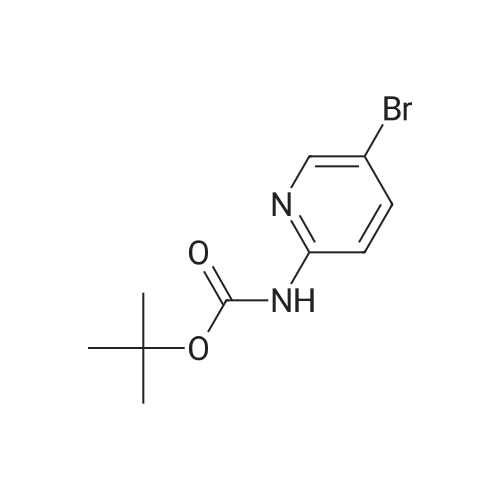
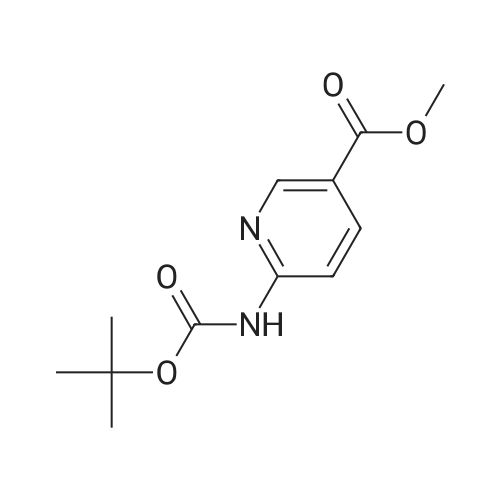
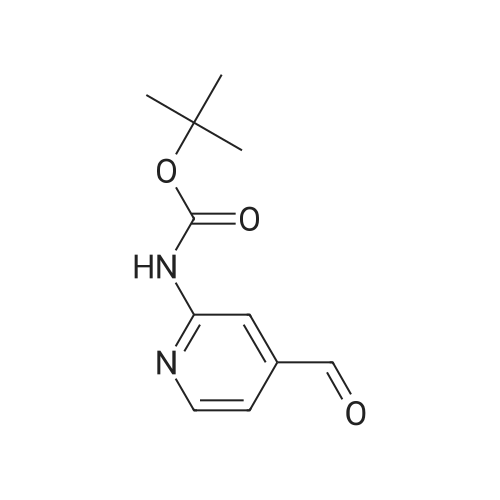
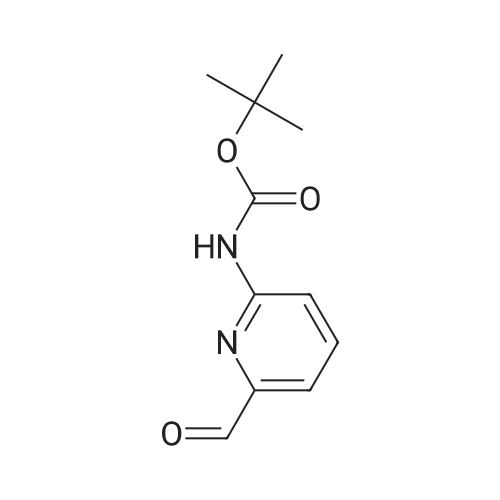
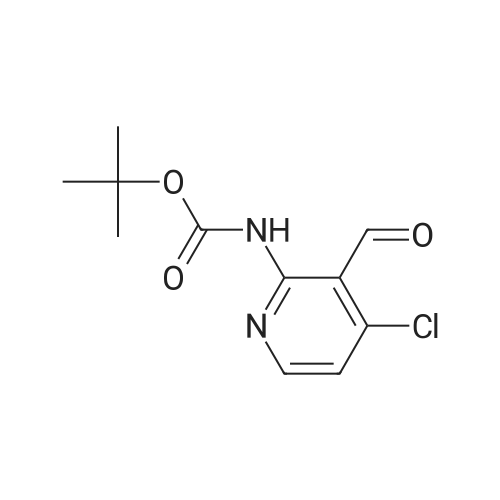
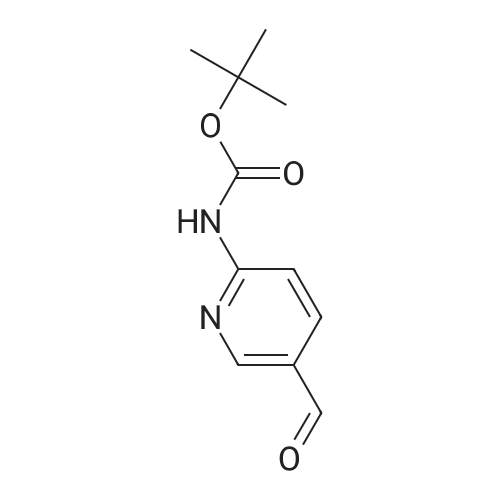
Step B 2-(t-Butoxycarbonylamino)-5-pyridinecarboxaldehyde To a solution of methyl-6-(tert-butoxycarbonylamino)-nicotinate (2.20 g, 8.72 mmol) in anhydrous THF (50 mL), cooled to -30 C. was added DIBAL-H (1.0M in hexane, 34. 8 mL, 34.8 mmol) dropwise. After 1 h, 40 mL of a saturated solution of Rochelle salts was added and stirred vigorously for 10 h. The volatiles were removed in vacuo and the aqueous layer was extracted with methylene chloride (3*50 mL). The organic layer was washed with water (1*50 mL) and brine (1*50 mL), dried over MgSO4, filtered, and concentrated in vacuo. The residue was used directly in the next step. To a solution of oxalyl chloride (4.12 g, 32.46 mmol) in methylene chloride (30 mL), cooled to -78 C., was added DMSO (2.54 g, 32.46 mmol) dissolved in methylene chloride (9 mL) dropwise. After 20 minutes, the crude alcohol (1.82 g, 8.11 mmol) dissolved in 35 mL of methylene chloride was added followed by triethylamine (4.92 g, 48.66 mmoL). The ice bath was removed and the reaction stirred for 1 hr at rt, then water (30 mL) was added. The layers were separated and the aqueous layer was extracted with methylene chloride (2*50 mL). The organic layer was washed with water (1*50 mL) and brine (1*50 mL), dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography (30*150 mm column of SiO2, EtOAc/methylene chloride 1:11) to give the title compound as a colorless solid: 1 H NMR (400 MHz, CDCl3) d 1.55 (s, 9H), 7.79 (br s, 1H), 8.13-8.14 (m, 2H), 8.71 (dd, J=1.1 and 1.8 Hz, 1H), 9.96 (s, 1H). |
|
With diisobutylaluminium hydride; triethylamine; In hexane; dichloromethane; water; dimethyl sulfoxide; |
Step B: 2-(t-Butoxycarbonylamino)-5-pyridinecarboxaldehyde To a solution of methyl-6-(tert-butoxycarbonylamino)-nicotinate (2.20 g, 8.72 mmol) in anhydrous THF (50 mL), cooled to -30C was added DIBAL-H (1.0M in hexane, 34.8 mL, 34.8 mmol) dropwise. After 1 h, 40 mL of a saturated solution of Rochelle salts was added and stirred vigorously for 10 h. The volatiles were removed in vacuo and the aqueous layer was extracted with methylene chloride (3 x 50 mL). The organic layer was washed with water (1 x 50 mL) and brine (1 x 50 mL), dried over MgSO4, filtered, and concentrated in vacuo. The residue was used directly in the next step. To a solution of oxalyl chloride (4.12 g, 32.46 mmol) in methylene chloride (30 mL), cooled to -78 C, was added DMSO (2.54 g, 32.46 mmol) dissolved in methylene chloride (9 mL) dropwise. After 20 minutes, the crude alcohol (1.82 g, 8.11 mmol) dissolved in 35 mL of methylene chloride was added followed by triethylamine (4.92 g, 48.66 mmoL). The ice bath was removed and the reaction stirred for 1 hr at rt, then water (30 mL) was added. The layers were separated and the aqueous layer was extracted with methylene chloride (2 x 50 mL). The organic layer was washed with water (1 x 50 mL) and brine (1 x 50 mL), dried over MgSO4, filtered, and concentrated in vacuo. The residue was purified by flash column chromatography (30 x 150 mm column of SiO2, EtOAc/methylene chloride 1:11) to give the title compound as a colorless solid: 1H NMR (400 MHz, CDCl3) d 1.55 (s, 9H), 7.79 (br s, 1H), 8.13-8.14 (m, 2H), 8.71 (dd, J = 1.1 and 1.8 Hz, 1H), 9.96 (s, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping