| 95.3% |
With sulfuric acid; at 0 - 70℃; for 24.5h; |
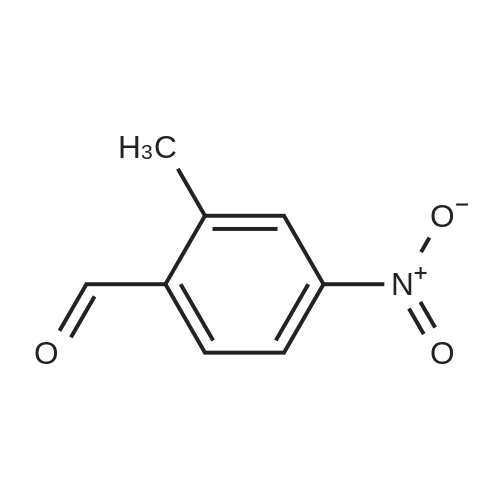
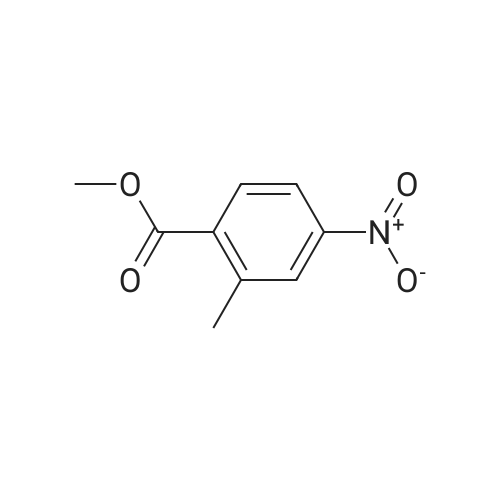
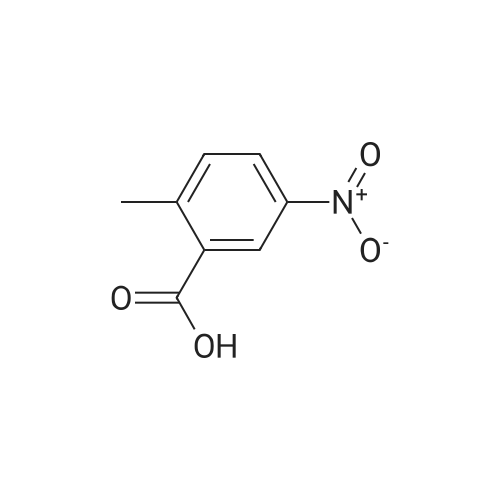
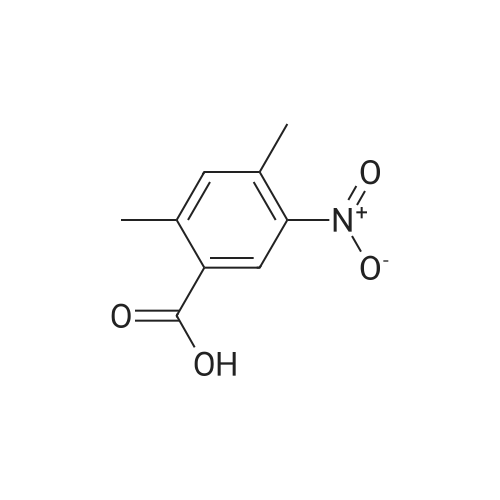
Step 1: Synthesis of 2-Methyl-4-nitro-benzoic acid methyl ester To a solution of 2-methyl-4-nitro-benzoic acid (3 g, 16.56 mmole) in 30 ml methanol was added at 0 C. sulfuric acid (95-98%, 10 ml) slowly for ? hr. The reaction mixture was stirred at 70 C. for 24 hrs. Mixture was cooled down, a solid precipitated out, then the mixture was concentrated, diluted with 50 ml water, stirred for 10 minutes, filtered off, the solid was washed with water, dried to afford 3.08 g of brown solid, 95.3% yield, pure by H NMR. |
| 94.65% |
With sulfuric acid; for 15h;Reflux; |
In a 250 mL, 3-neck round-bottomed flask, 2-methyl-4-nitrobenzoic acid (20 g, 93.8 mmol) was charged with methanol (100 mL) and sulfuric acid (4 mL). The reaction mixture was refluxed for 15 h. After completion, the reaction mixture was concentrated to obtain the crude product which was added water (200 mL) and basified to pH 7-8 with aqueous NaHC03 solution. Aqueous solution was extracted with dichloromethane (250 mL X 3), the organic layer was washed with saturated brine solution followed by drying over anhydrous sodium sulphate. The solvent was removed to obtain methyl 2-methyl-4- nitrobenzoate 1), 20 g; Yield: 94.65%. |
| 93% |
With thionyl chloride; at 0 - 80℃; for 2h; |
SOCl2 (2.6 g, 22.0 mmol) was slowly added to the solution of 2-methyl-4- nitrobenzoic acid (61.1) (2.0 g, 11.0 mmol) in MeOH (20 mL) at 0 C. The resulting mixture was heated at 80 C for 2 h. After cooling to room temperature, the reaction mixture was concentrated to afford methyl 2-methyl-4-nitrobenzoate (61.2) as a yellow oil (2.0 g, 93%). |
| 91.3% |
With thionyl chloride; at 12 - 20℃; for 12h; |
Thionyl chloride (4.3 g, 36.45 mmol) was added dropwise to a stirred solution of 18 (3 g, 16.57 mmol) in MeOH (25 mL) while maintaining the internal temperature below 12 C. When the addition was complete the mixture was left to stand at room temperature for 12 h to result a white precipitation. The mixture was filtered and the filtrate was concentrated under reduced pressure to afford white solid. The solid was washed with hexane and ethyl ether to afford 19 (2.95 g, 91.3%); mp 153.7-154.4 C (lit.39 mp 153-154 C); Rf 0.43 (hexane/EtOAc 3:1); 1H NMR (CDCl3) delta 2.69 (s, 3H, CH3), 3.95 (s, 3H, COOCH3), 8.02-8.11 (m, 3H, C6H3). |
| 90.9% |
With sulfuric acid; at 20 - 60℃; |
EXAMPLE 75 2-Methyl-4-nitro-benzoic acid methyl ester (1) In accordance with general method S, 5.00 g (27.60 mmol) of 2-methyl-4-nitro-benzoic acid are dissolved in 20 ml of methanol, with heating, and 6 ml of concentrated sulfuric acid are added. The mixture is refluxed for 5 h. Yield: 4.90 g (90.9%); melting point: 73.6 C. C9H9NO3(Mr=195.18); GC (method 1) 9.55 min 1H-NMR (CDCl3) in ppm: 8.19-8.09 (m, 2H, aryl H), 7.99 (d, 1H, J=8.52 Hz, aryl H), 3.87 (s, 3H, -OCH3), 2.59 (s, 3H, -CH3) 13C-NMR (CDCl3) in ppm: 166.35 (-C=O), 159.29 (C4), 141.79 (C2), 135.14 (C1), 131.43 (C6), 126.06 (C3), 120.48 (C5), 52.37 (-OCH3), 21.50 (-CH3) IR (ATR) (cm-1): 1722, 1523, 1432, 1348, 1260, 1081, 896, 821, 786, 730 MS m/z (%): 195 (70), 164 (100), 134 (21), 118 (29), 63 (15). General Method SFor esterification of the acid group of the starting compound 2-methyl-4-nitro-benzoic acid, the stated amount is dissolved in methanol in a 100 ml one-necked flask at room temperature, concentrated H2SO4 is added and the mixture is refluxed at approx. 60 C. for 6 h. The excess alcohol is removed in vacuo, the residue is taken up in EtOAc and the mixture is deacidified repeatedly with 20% strength sodium hydroxide solution. After drying (Na2SO4), the combined organic phases are concentrated in vacuo. |
|
With hydrogenchloride; In 1,4-dioxane; for 48h;Heating / reflux; |
Step 2:; A solution of the acid 11-2 from step 1 (3.60 g, 15.6 mmol) in MeOH (30 mL) and HCI (4N HCI in dioxane, 2.0 mL) was heated to reflux for 48 h. The solvent was evaporated to dryness under vacuum and the residue obtained was re-dissolved in EtOAc (200 mL). The solution was washed with aqueous saturated NaHC03 (100 mL) and brine (100 mL), dried over anhydrous MgS04 and evaporated to dryness to give the ester intermediate 11-3 as a yellow-colored solid (2.38 g). This material was used in step 3 without purification. |
|
With sulfuric acid; at 55℃; for 50h; |
2-Methyl-4-nitrobenzoic acid methyl ester; [697] H2SO4 (98%, 0.030ml) was added dropwise into the MeOH (30mL) solutionof 2-methyl-4-nitrobenzoic acid at rt. The mixture was then heated at 55C. After 50h, thesolvent was removed in vacua and the residue was partitioned between EtOAc and H2O(25mL each). The aqueous layer was extracted with another portion of EtOAc (25mL). Thecombined extracts were washed with saturated NaHC03 (25mL), H2O (2 x 25mL), and brine(25mL), dried over MgS04, filtered and concentrated in vacua to give the title compound aslight-yellow solid. 'H NMR (CDC13,400 MHz): 5 = 2.69 (s, 3 H), 3.95 (s, 3 H), 8.03 - 8.12(m, 3 H). MS (ES+): m/z 196.25 (100) [MH4]. HPLC: /R= 3.29 min (ZQ2000, polar_5 min). |
|
|
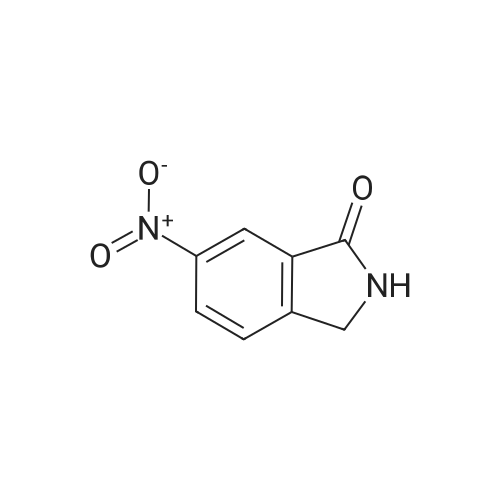
Preparation of 5-amino-2,3-dihydro-1H-isoindol-1-one A mixture of 2-methyl-4-nitrobenzoic acid (0.906 g, 5 mmol) in sulfurous dichloride (5.95 g, 50 mmol) was refluxed for 1 hour and concentrated. The residue was dissolved in methanol (30 mL, 741 mmol) and triethylamine (2 g, 19.76 mmol) was added. The mixture was stirred at room temperature for 1 hour, concentrated, and partitioned between ethyl acetate and water. The organic layer was separated, dried over MgSO4, and filtered through Magnesol to give 862 mg of methyl 2-methyl-4-benzoate as a tan solid. |
|
sulfuric acid; In water; at 20 - 55℃; |
Example 44Preparation of 5-[(2-((lS.2SV2-?-('5-chloropyrimidin-2-yl)piperidin-4- yl] cyclopropyl ) ethvDaminolisoindolin- 1 -oneStep 1 : methyl 2-methyl-4-nitrobenzoateSulfuric acid (6 ml, 113 mmol) was added to drop wise to a solution of the 2-methyl~4- nitrobenzoic acid (5 g, 27.6 mmol) in MeOH (138 ml) at RT. After the addition was complete the mixture was heated to 55 C overnight. The volatiles were removed in vacuum and the residue partitioned between EtOAc (100 ml) and water (100 ml). The layers were separated and aqueous phase extracted with EtOAc (2x100 ml). The organic fractions were combined, washed with saturated sodium bicarbonate (aqueous, 100ml), brine, dried over Na2SO4, filtered and the volatiles removed in vacuum to give the titled compound. |
|
With sulfuric acid; for 10h;Reflux; |
2-methyl-4-nitrobenzoic acid (1.81g, 10.0mmol) was dissolved in CH3OH (10mL), concentrated sulfuric acid (2.0mL, 3.7eq).The reaction system was heated to reflux, the reaction 10h.Completion of the reaction, cooled to room temperature, and thereto was added Na2CO3And sulfuric acid, and then filtered. The mother liquor after removal of methanol, to give compound I The crude yield of 89%, can be used directly in the next reaction. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping