|
With potassium carbonate; In ethyl acetate; N,N-dimethyl-formamide; |
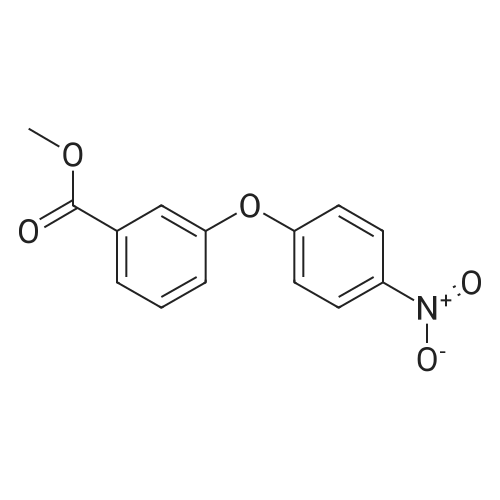
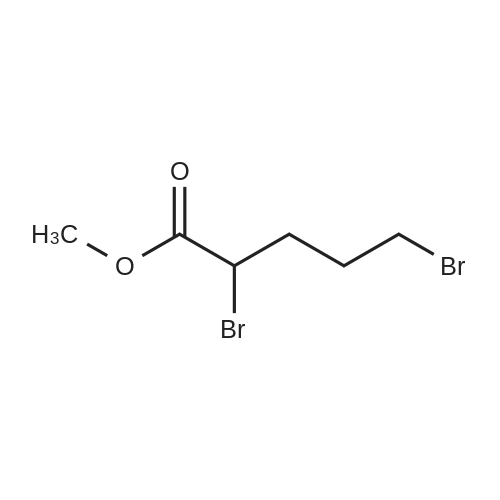
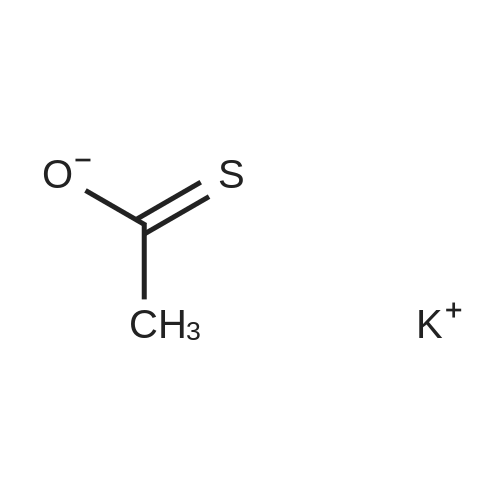
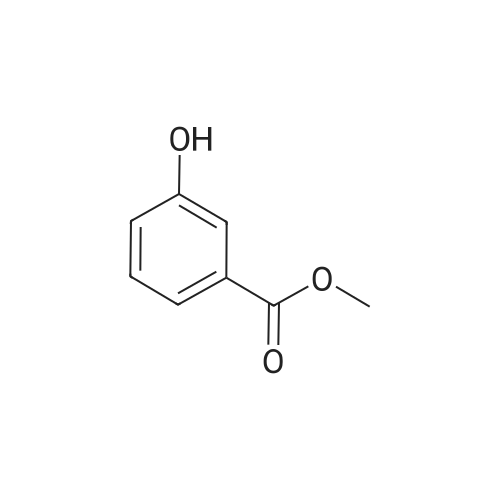
Methyl 3-(4-acetylthio-1-methoxycarbonyl-butoxy)-benzoate XV To a solution of <strong>[50995-48-7]methyl 2,5-dibromopentanoate</strong> XIII (22.00 g, 80.3 mmol) and methyl 3-hydroxybenzoate XIV (10.18 g, 66.9 mmol) in DMF (80 mL) was added K2CO3 (12.94 g, 93.7 mmol) at room temperature. The mixture was stirred at room temperature under N2 for 12 hours, then heated at 70° C. for 1 hour. Potassium thioacetate (22.93 g, 200.8 mmol) was added to the mixture, which was heated at 70° C. for 2 hours. The mixture was allowed to cool to room temperature and was diluted with EtOAc (1000 mL). The mixture was washed with H2O (3*300 mL) and brine (2*300 mL). The organic layer was dried over MgSO4, filtered and concentrated. The crude product was purified by flash chromatography (gradient elution: 10percent to 20percent EtOAc/hexanes) to afford methyl 3-(4-acetylthio-1-methoxycarbonyl-butoxy)-benzoate XV (8.40 g, 24.7 mmol, 37percent) as a yellow oil: Rf 0.26 (hexanes/EtOAc, 4:1): 1H NMR (CDCl3) delta1.73-1.88 (m, 2H), 2.01-2.08 (m, 2H), 2.32 (s, 3H), 2.93 (t, J=7.2 Hz, 2H), 3.75 (s, 3H), 3.89 (s, 3H), 4.69 (t, J=6.2 Hz, 1H), 7.07 (dm, J=8.0 Hz, 1H), 7.33 (t, J=7.9 Hz, 1H) 7.50 (m, 1H), 7.65 (dm, J=7.7 Hz, 1H); 13C NMR (CDCl3) delta25.3, 28.3, 30.5, 31.4, 52.1, 52.2, 75.8, 115.4, 120.0, 122.8, 129.5, 131.4, 157.5, 166.4, 171.3, 195.4. |
|
With potassium carbonate; In ethyl acetate; N,N-dimethyl-formamide; |
Methyl 3-(4-acetylthio-1-methoxycarbonyl-butoxy)-benzoate XV To a solution of <strong>[50995-48-7]methyl 2,5-dibromopentanoate</strong> XIII (22.00 g, 80.3 mmol) and methyl 3-hydroxybenzoate XIV (10.18 g, 66.9 mmol) in DMF (80 mL) was added K2CO3 (12.94 g, 93.7 mmol) at room temperature. The mixture was stirred at room temperature under N2 for 12 hours, then heated at 70° C. for 1 h. Potassium thioacetate (22.93 g, 200.8 mmol) was added to the mixture, which was heated at 70° C. for 2 hours. The mixture was allowed to cool to room temperature and was diluted with EtOAc (1000 mL). The mixture was washed with H2O (3*300 mL) and brine (2*300 mL). The organic layer was dried over MgSO4, filtered and concentrated. The crude product was purified by flash chromatography (gradient elution: 10percent to 20percent EtOAc/hexanes) to afford methyl 3-(4-acetylthio-1-methoxycarbonyl-butoxy)-benzoate XV (8.40 g, 24.7 mmol, 37percent) as a yellow oil: Rf 0.26 (hexanes/EtOAc, 4:1): 1H NMR (CDCl3) delta1.73-1.88 (m, 2H), 2.01-2.08 (m, 2H), 2.32 (s, 3H), 2.93 (t, J=7.2 Hz, 2H), 3.75 (s, 3H), 3.89 (s, 3H), 4.69 (t, J=6.2 Hz, 1H), 7.07 (dm, J=8.0 Hz, 1H), 7.33 (t, J=7.9 Hz, 1H), 7.50 (m, 1H), 7.65 (dm, J=7.7 Hz, 1H); 13C NMR (CDCl3) delta25.3, 28.3, 30.5, 31.4, 52.1, 52.2, 75.8, 115.4, 120.0, 122.8, 129.5, 131.4, 157.5, 166.4, 171.3, 195.4. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping