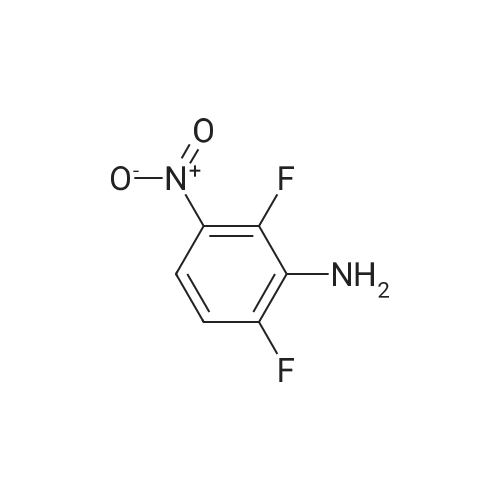
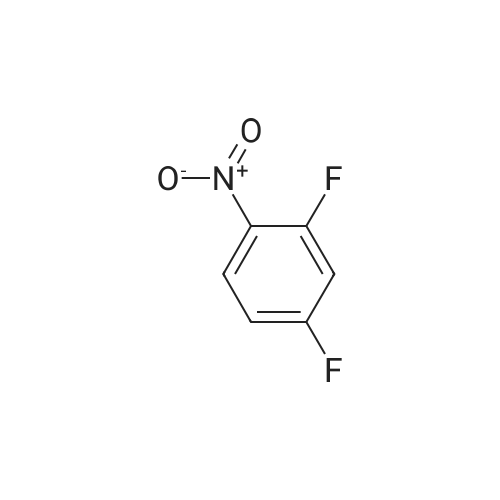
| 61% |
With potassium tert-butylate; In dimethyl sulfoxide; at 20℃; for 18.5h; |
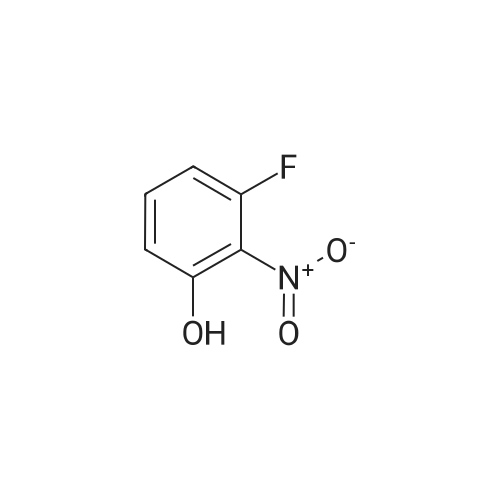
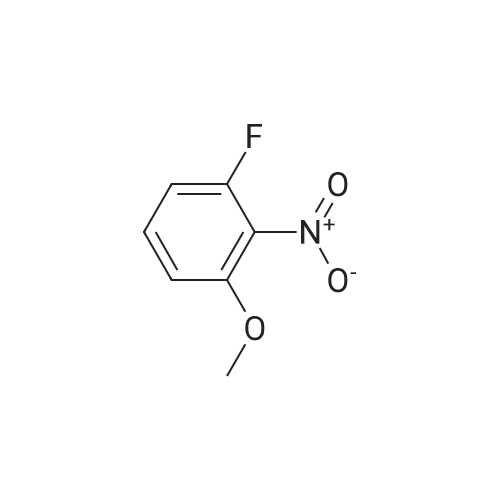
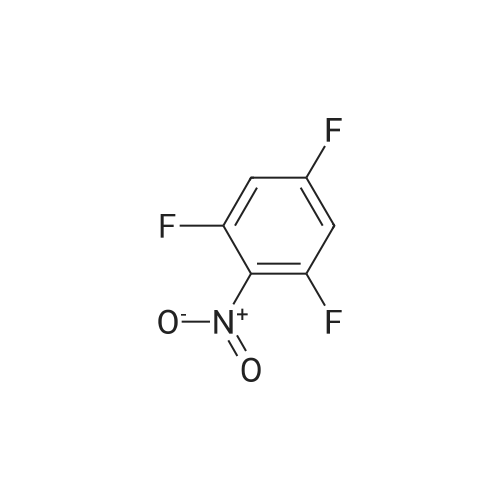
A solution of potassium TERT-BUTOXIDE (1.23 g, 11 mmol) in 25 mL of anhydrous DMSO was stirred at room temperature for 30 minutes and treated with 1, 3-difluoro-2-nitrobenzene (1.59 g, 10 mmol). After 18 hours, the mixture was diluted with 150 mL of 1 N aqueous sulfuric acid and extracted with three 50 mL portions of diethyl ether. The combined organic layers were washed with water, dried over sodium sulfate, filtered, and concentrated in vacuo. The residue was dissolved in 50 mL of trifluoroacetic acid. After 30 minutes at room temperature, the mixture was concentrated in vacuo, treated with 50 mL of 1 N aqueous sodium hydroxide, and extracted with three 30 mL portions of diethyl ether. The aqueous layer was acidified with 1 N aqueous sulfuric acid and extracted with two 50 mL portions of dichloromethane. The combined dichloromethane layers were washed with water, dried over sodium sulfate, filtered, and concentrated in vacuo to give 1.3 g of 3-fluoro-2-nitrophenol as orange oil (61% yield). An solution of 3-fluoro-2-nitrophenol (1.13 g, 7.2 mmol) and pyridine (0.65 mL, 8 mmol) in 15 mL of anhydrous dichloromethane was cooled in an ice bath and treated with a solution of triflic anhydride (1.33 mL, 7.9 mmol) in 3 mL of anhydrous dichloromethane. After 4 hours, the reaction mixture was diluted with 100 mL of dichloromethane, washed with two 30 mL portions of saturated aqueous sodium bicarbonate, two 30 mL portions of 1 N aqueous sulfuric acid, and two 30 mL portions of water, dried over sodium sulfate, filtered, and concentrated in VACUA TAO give 2 g of the title product as a light brown oil (96% yield). |
|
|
To a mixture of potassium tert-butoxide (7.76 g) in dimethyl sulfoxide (150 ml) was added <strong>[19064-24-5]1,3-difluoro-2-nitrobenzene</strong> (10 g), and stirred at room temperature for 18 h. The reaction mixture was diluted with 1N hydrochloric acid, and extracted with 1,1'-oxydiethane. The combined organic layer was washed with water, dried over anhydrous magnesium sulfate and concentrated. The residue was dissolved in trifluoroacetic acid (100 ml), and stirred at room temperature for 1 h. The mixture was concentrated, and the residue was diluted with 1N sodium hydroxide, washed with 1,1'-oxydiethane. The aqueous layer was acidified with 1N hydrochloric acid, and extracted with 1,1'-oxydiethane. The combined organic layer was washed with water, dried over anhydrous magnesium sulfate and concentrated to give the title compound (7.59 g) as crude. The crude compound was used to next reaction without further purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping