|
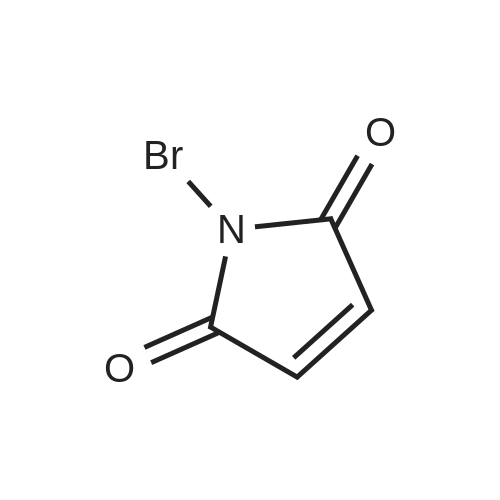
With N-Bromosuccinimide; dibenzoyl peroxide; In tetrachloromethane; at 85℃; for 16h;Irradiation; |
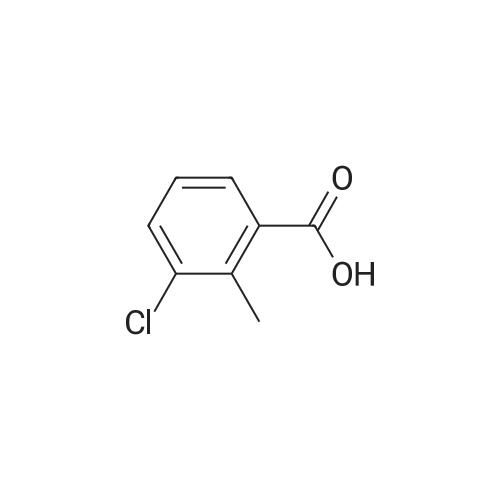
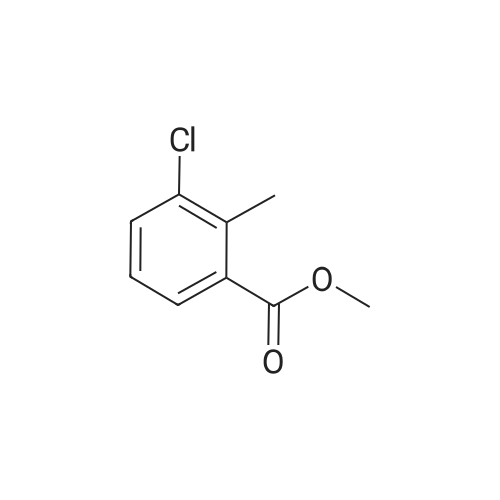
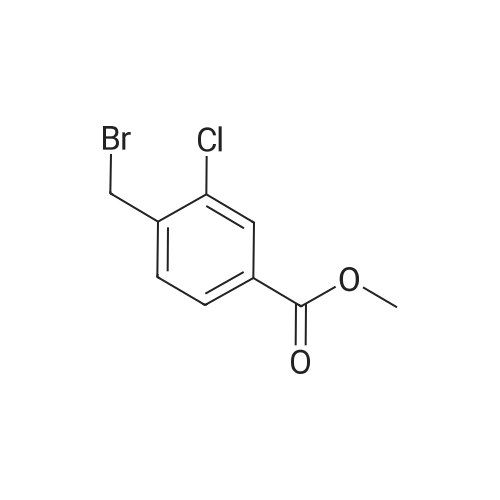
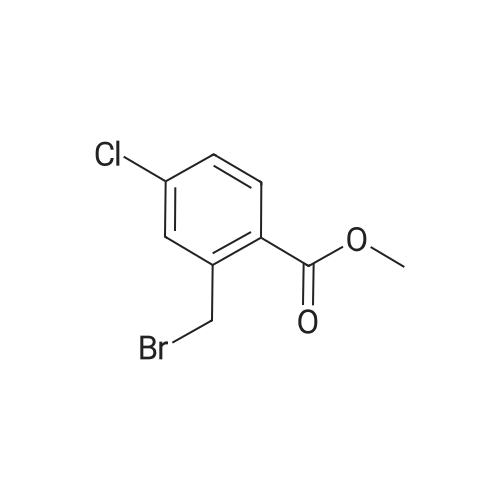
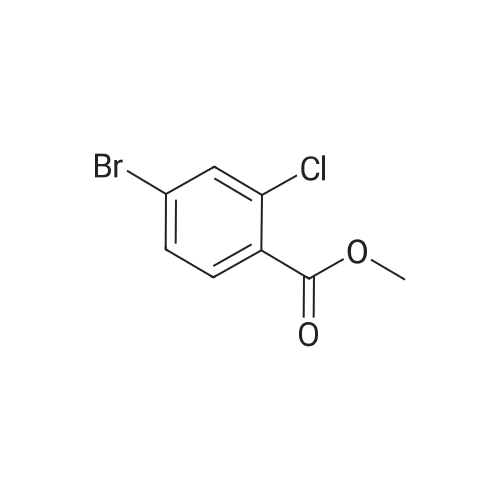
A. To a suspension of 3-chloro-2-methylbenzoic acid methyl ester (5.62 g, 30.4 MMOL), and N-bromosuccinimide (5.94 g, 33.4 MMOL) in carbon TETRACHLORIDE (200 mL) was added benzoyl peroxide (800 mg, 3.30 MMOL). The resulting suspension was immersed in an oil bath held at 85C, and illuminated with a 300W halogen worklight. After stirring for 16 hours with heat and illumination the reaction mixture was allowed to cool to ambient temperature, filtered to remove the insoluble succinimide, and concentrated under reduced pressure to afford 2- bromomethyl-3-chlorobenzoic acid methyl ester as a yellow semi-solid. This crude material was carried on to the next step without purification. |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In tetrachloromethane; for 3h;Heating / reflux; |
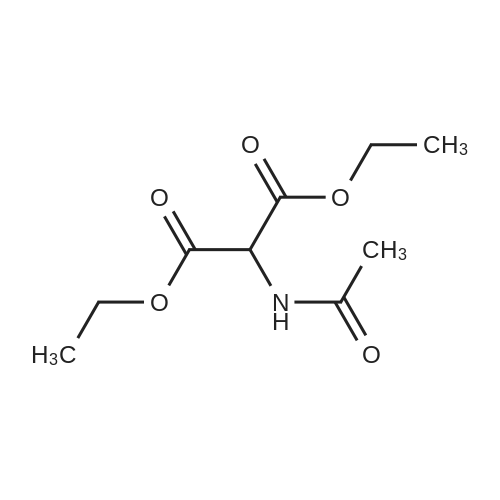
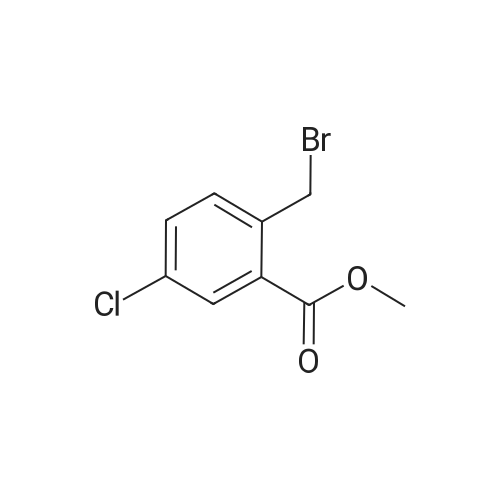
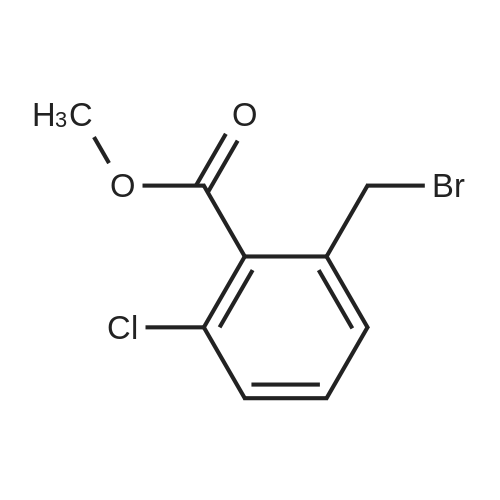
Example 28 1 - [ (5-CHLORO-1, 2,3, 4-tetrahydroisoquinolin-3-yl) METHYL]-1-METHYL-1, 2- dihydrospiro [indole-3, 4'-piperidine] (A) 5-CHLORO-L-OXO-1, 2,3, 4-tetrahydroisoquinoline-3-carboxylic acid To a stirred suspension of 3-chloro-2-methylbenzoic acid (10.00 g, 58.6 mmol) in methanol (100 mL) was added dropwise thionyl chloride (8.5 mL, 117.2 mmol) at 0 C. The reaction mixture was refluxed for 2 h. After cooling down to room temperature, the reaction mixture was concentrated in vacuo. The residue was dissolved in ethyl acetate (300 ML), washed with 1N NaOH solution (100 mL), water (100 mL) and brine (100 ML), dried (NA2S04), filtered, and concentrated to give 10.86 g of methyl ester as colorless oil. A mixture of this ester (10.80 g, 58.5 mmol), N-bromosuccinimide (10.45 g, 64.4 mmol), and benzoyl peroxide (0.71 g, 2.9 mmol) in carbon tetrachloride (70 mL) was refluxed for 3 h. After cooling down to room temperature, the reaction mixture was filtered and the filtrate wad concentrated to give 16.30 g of methyl 3-chloro-2-bromomethylbenzoate as a colorless oil. To a stirred suspension of NaH (2.45 g, 61.3 mmol) in DMF (50 mL) was added dropwise a solution of diethyl acetamidomalonate (12.10 g, 55.7 mmol) in DMF (50 mL) at room temperature. After 15 min stirreing, a solution of methyl 3-chloro-2- bromomethylbenzoate (16.3 g, 58. 5 mmol) in DMF (50 mL) at room temperature. After 18 h stirring, the reaction mixture was concentrated. The residue was dissolved in ethyl acetate (800 ML), washed with water (200 mL x 2) and brine (200 mL), dried (NA2S04), filtered, and concentrated to give 21.5 g of yellow solid, which was purified by silica gel column chromatography (hexane/ethyl acetate: 2/1 to 111) to afford 14.33 g (64.3 %) of coupling product as a white solid. A mixture of this solid (13.90 g, 34.7 mmol) and 47 % HBr (160 mL) was refluxed for 24 h. After cooling down to room temperature, the solid formed was collected by filtration and dried togive 6.16 g (78.6 %) of title compound as a cream color solid. LHNMR (300MHZ, DMSO-D6) O8. 26 (1H, d, J = 4. 0 HZ), 7.85 (1H, dd, J = 1.1, 7.7 Hz), 7.64 (1H, dd, J = 1. 3, 8. 1 HZ), 7.39 (1H, dd, J = 7. 9,7. 9 HZ), 4.34-4. 28 (1H, m), 3.42-3. 36 (1H, overlapped with water peak at 3.36 ppm), 3.26 (1H, dd, J = 6.8, 16.9 Hz). |
|
With N-Bromosuccinimide; dibenzoyl peroxide; In 1,2-dichloro-ethane; at 80℃; for 12h; |
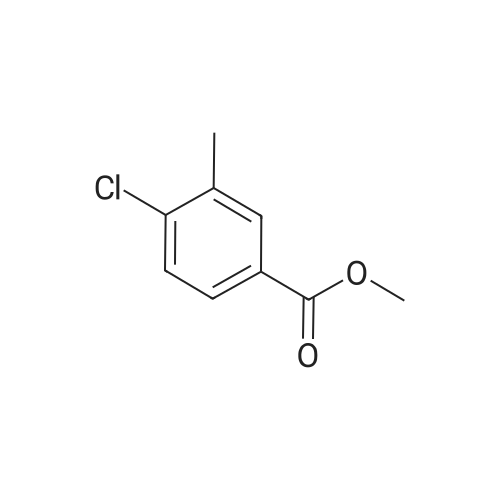
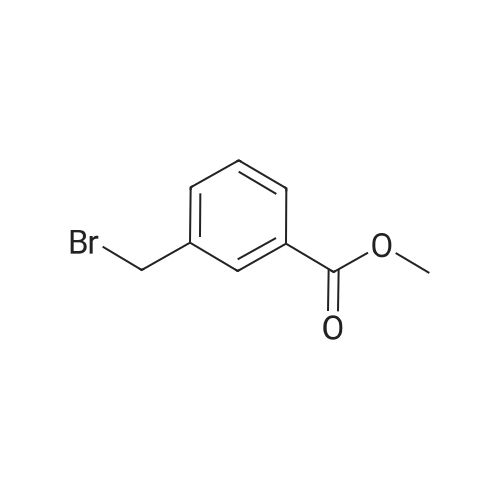
General procedure: A mixture of methyl 3-bromo-2-methylbenzoate (500 mg, 3.3 mmol), NBS (770.4 mg, 4.3 mmol), and di-benzoyl peroxide (BPO, 80.7 mg, 0.3 mmol) in 1,2-dichloroethane (10 mL) was heated at 80 C for 12 h. The reaction mixture was cooled to room temperature, and the precipitated solid was removed by filtration and washed with ethers (10 mL). The filtrate was concentrated in vacuo and the residue was partitioned between 2 N NaHCO3 (15 mL) and ethers (15 mL). The organic layer was separated, dried over NaSO4, filtered and concentrated to give a crude product (683.2 mg, 89.6%), which was used in the next step reaction without further purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping