| 16%; 18% |
|
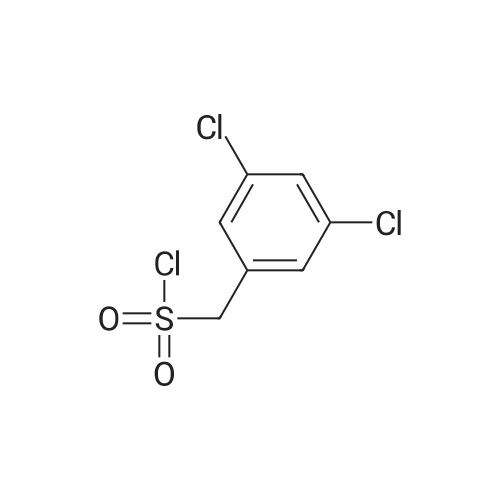
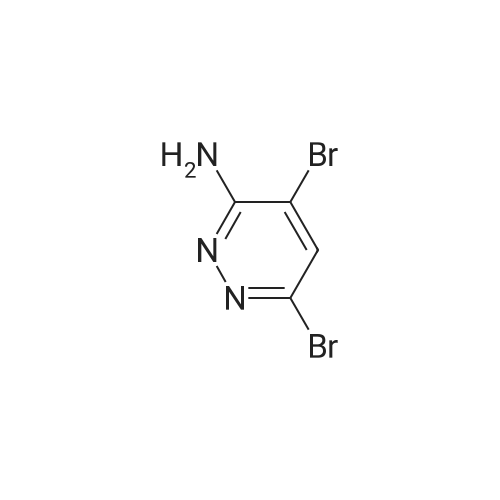
Toa mixture of 6-iodopyridazin-3-amine (500 mg, 2.26 mmol), NaHC03(230 mg, 2.71 mmol) in MeOH (5 mL) was added bromine (117 μ, 2.26 mmol)dropwise. The resulting mixture was stirred at room temperature for 16 hrs. Thesolution was filtered and the filtrate concentrated in vacuo. The residue wasdissolved in water, and the product extracted with EtOAc (3 times). The organiclayers were combined, dried ( a2S04) and concentrated invacuo to give a dark red solid which was purified by flash silicachromatography (eluent: 20% EtOAc :Hexane) to give a 60:40 mixture of the titlecompounds as an off white solid (250 mg); H NMR (400 MHz, CDC13) δ5.49 (s, 4H), 7.66 (s, 1H), 7.81 (s, 1H)Toa stirred solution of a mixture of 4,6-dibromopyridazin-3-amine and 4-bromo-6-iodopyridazin-3-amine (10 g, compounds not separated in previous step,estimated 34 mmol) in methanol (90 mL) was added solid sodium methoxide (3.6 g,67 mmol) at room temperature and the reaction mixture stirred at 90C for16hrs. More sodium methoxide was added regularly until all starting materialhad been consumed. The cooled solution was concentrated in vacuo and theresidue poured into water (200 mL). The resulting solution was extracted withEtOAc three times and the organic layers were combined, dried (MgSC^), andconcentrated in vacuo. The residue was purified by silica column chromatography(eluent: chloroform: methanol (98:0.2 to 90:10) to afford the title mixture ofmethoxy ethers (3.0g, taken into next steps without further purification); HNMR (400 MHz, CDC13) δ 3.90 (s, 3H), 3.92 (s, 3H), 5.05 (m, 3H),6.75 (s, 1H), 6.91 (s, 1H).To a stirred solution of (6-bromo-4-methoxypyridazin-3-amine and 6-iodo-4-methoxy- pyridazin-3 -amine (0.8 g, mixture not separated in previous step, estimated 3.92 mmol) in pyridine (9 mL) was added <strong>[163295-70-3](3,5-dichlorophenyl)methanesulfonyl chloride</strong> (1.02 g, 3.92 mmol) at room temperature and the mixture stirred for 16 hrs. Water was then added and the mixture extracted with EtOAc (3x 150 mL). The combined organic layers were washed with more water and brine, then concentrated and purified by flash silica chromatography (eluent: 1% MeOH:DCM) to afford a mixture of methoxy ethers (250mg). To this mixture in DCM (5 mL) was added neat boron tribromide (166 μ, 1.76 mmol) and the mixture stirred for 3h before being diluted with DCM and neutralised with saturated NaHC03 (pH 7). The phases were separated and the aqueous phase extracted with DCM and EtOAc. The combined organic layers were dried (MgSC^), the mixture filtered and the filtrate concentrated to dryness to yield an oil which was purified by automated reverse phase HPLC (low pH method) to afford 1 -(3,5-dichlorophenyl)-N-(4- hydroxy-6-iodopyridazin-3-yl)methanesulfonamide (22 mg, 16%) and -(6-bromo-4- hydroxypyridazin-3-yl)-l-(3,5)-dichlorophenyl)methanesulfonamide (22 mg, 18%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping