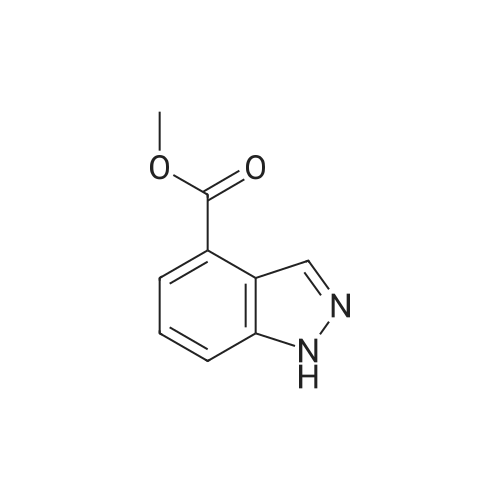
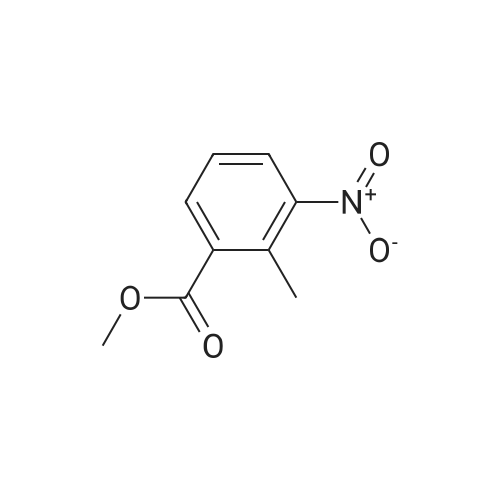
| 69% |
With acetic acid; sodium nitrite; In water; |
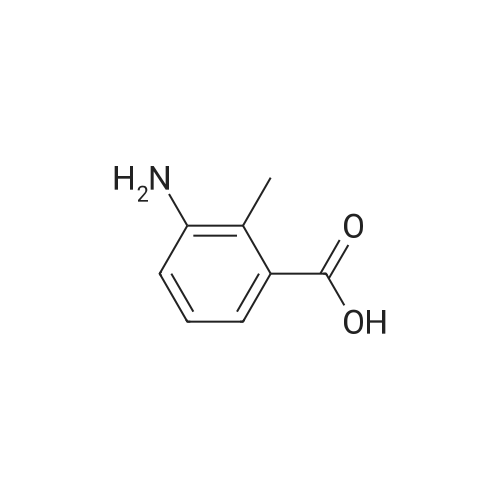
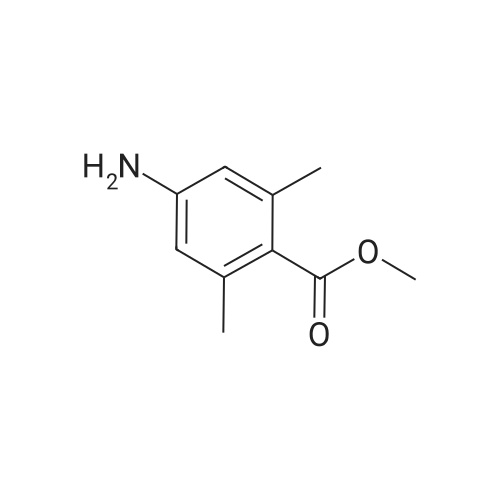
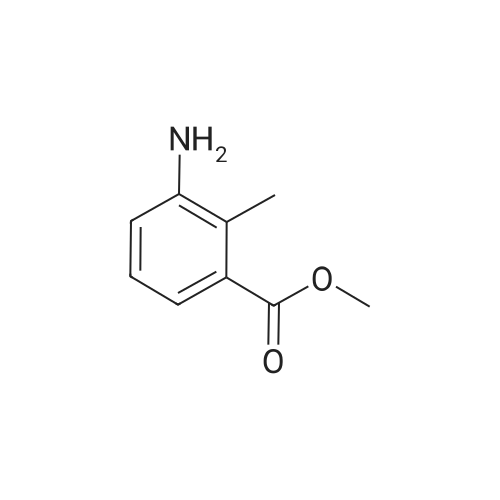
Methyl-3-amino-2-methyl benzoate (0.3 g, 1.96 mmol) was stirred in aqueous NaNO2 (0.62 g, 2 mmol) followed by addition of 7 mL dilute glacial acetic acid in water (7 mL, 3 mmol) (0.2:10). The reaction mixture was allowed to stir for 4-6 hrs. The mixture was then extracted with EtOAc and washed with water (2×10 mL). The organic layer was dried over Na2SO4, concentrated and subjected to column chromatography to obtain yellow powder. The yield was 0.22 g, 69%; m.p. 153-155 C. 1H NMR (400 MHz CDCl3, TMS, ppm) delta 2.55 (s, 3H), 7.37 (t, 1H, J=14.32 Hz), 7.53 (s, 1H), 7.67 (d, 1H, J=8.12 Hz), 7.82 (d, 1H, J=8.16 Hz), 9.79 (s, 1H). |
| 59% |
|
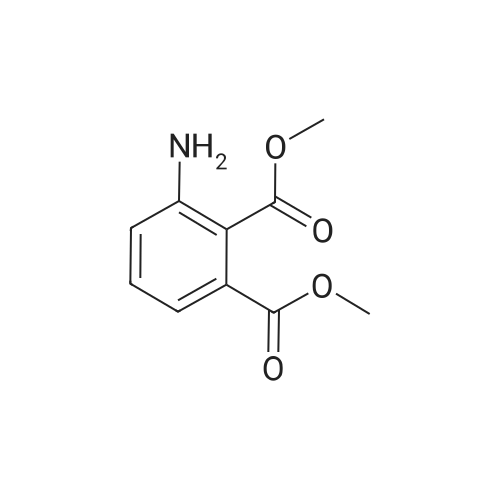
Reference Example 1 methyl 1H-indazole-4-carboxylate A mixture of methyl 3-amino-o-toluate (100 g, 605 mmol), a solution of ammonium tetrafluoroborate (83.0 g, 787 mmol) in water (600 mL) and concentrated hydrochloric acid (121 mL, 3.93 mmol) was cooled to 0C, and a solution of sodium nitrite (41.8 g, 605 mmol) in water (88 mL) was added dropwise to the mixture over 25 min. This mixture was stirred for 35 min, and the resulting solid was collected by filtration. This solid was washed with water, methanol and diethyl ether, dried under nitrogen atmosphere, and added to a solution of potassium acetate (65.4 g, 666 mmol) and 18-crown-6 (4.50 g, 17.0 mmol) in chloroform (1.37 L). The resulting mixture was stirred at room temperature for 2 hr, and water (700 mL) was added. The partitioned organic layer was washed with water, and dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The residue was triturated with hexane, and collected by filtration to give the title compound (63.0 g, yield 59%). 1H-NMR (CDCl3) delta: 4.03 (3H, s), 7.47 (1H, dd, 8.4, 7.2 Hz), 7.73 (1H, d, J = 8.4 Hz), 7.96 (1H, d, J = 7.2 Hz), 8.61 (1H, s), MS (ESI+): 177 (M+H). |
|
|
Step 3: methyl 1 H-indazole-4-carboxylate; To a cold (0-5 C) solution of methyl-3-amino-2-methyl benzoate (16.6 mL, 19.0 g, 0.12 mol) in chloroform (200 mL) was added dropwise acetic anhydride (24.8 mL, 0.26 mol) followed by stirring for 5 minutes. The resulting mixture was allowed to warm to room temperature and stirred for 1 hour and then was added potassium acetate (3.35g, 0.034 mol) and isoamyl nitrite (33.0 mL, 0.25 mol) and heated under reflux for 20 hours. The mixture was cooled to room temperature and solvent was evaporated to yield a brown solid. Water was added to the solid, followed by evaporation to yield a solid residue. The residue was treated with concentrated hydrochloric acid and the resulting mixture was heated at 500C for 2 hours. After cooling with an ice bath, the solution was basified to pH 14 with a 50% potassium hydroxide solution. The resulting solid was collected by filtration, washed with water and dried to yield 17.8 g of methyl 1 H-indazole-4-carboxylate as a beige solid. MS: 177.0 [M+H]. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping