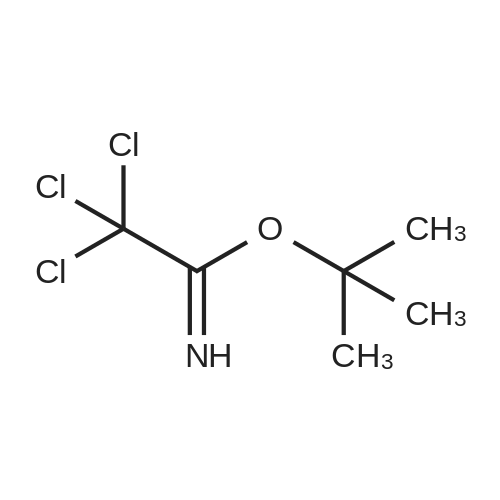
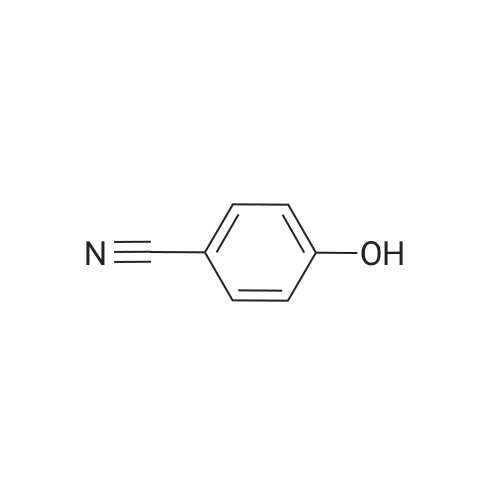
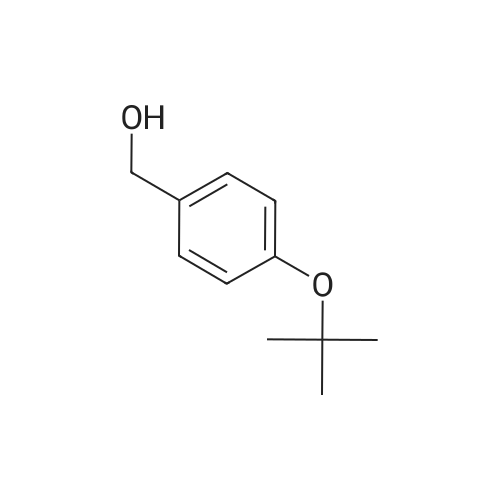
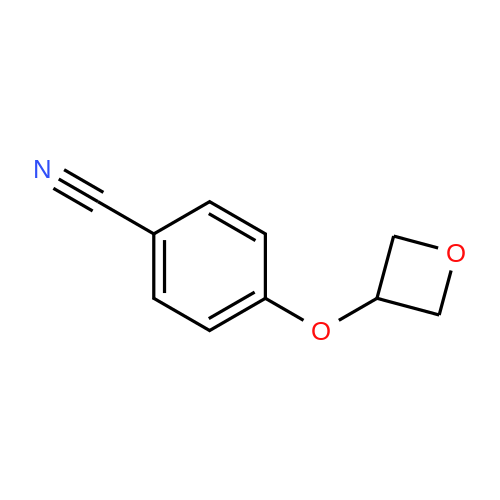
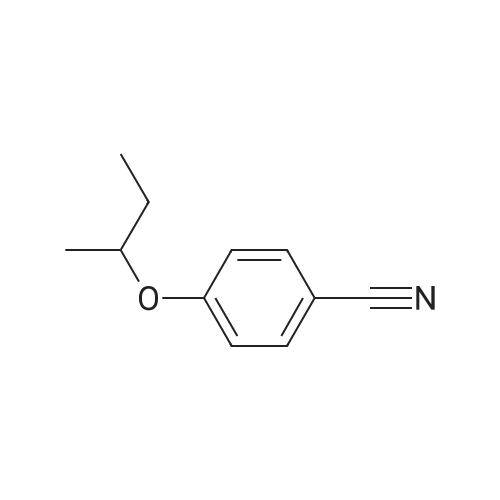
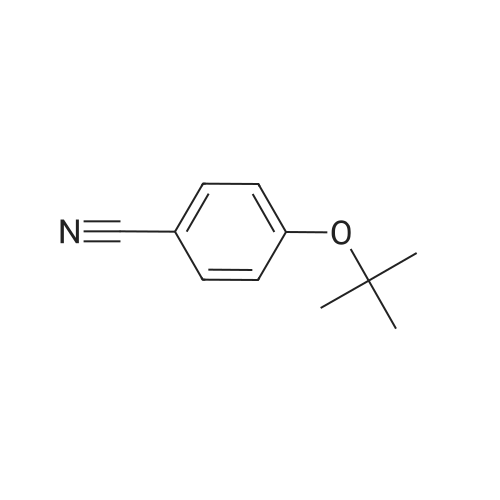
| 95% |
With lithium aluminium tetrahydride; In tetrahydrofuran; diethyl ether; for 3h;Reflux; |
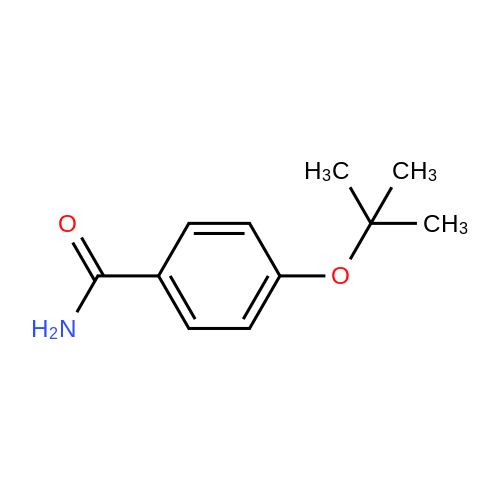
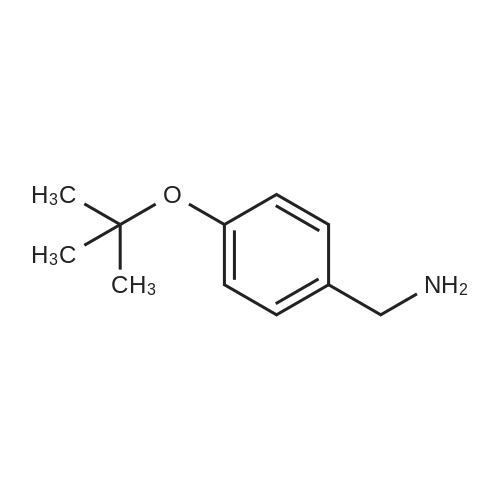
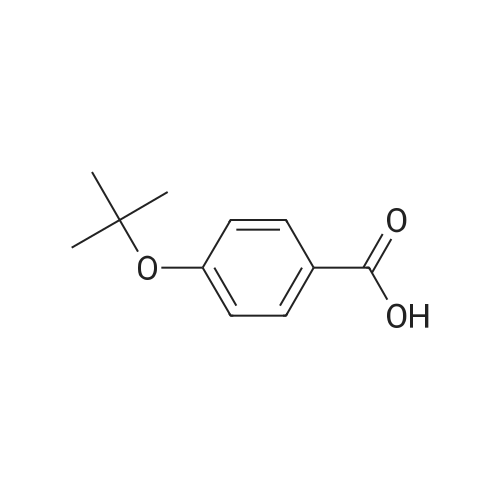
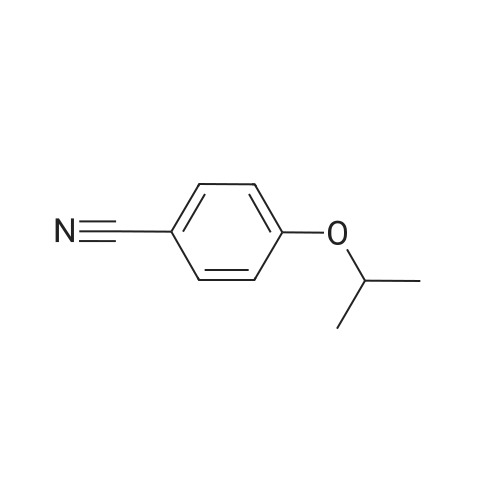
3.75 g of Mg(ClO4)2 (16.8 mmol, 0.1 equiv) in a 500 mL round-bottom flask was heated under vacuum at 130 C for 2 h and cooled to room temperature under argon. Then, 20 g of 4-cyanophenol (168 mmol, 1 equiv) in 200 mL of DCM was added, followed by 88.8 mL of Boc2O (387 mmol, 2.3 equiv). The resulting mixture was refluxed for at least 3 h and the reaction was monitored by TLC (20 % Et2O/hexanes). Once no more progression observed, the mixture was diluted in 300 mL of water and extracted twice with DCM. The organic layers were combined, dried with MgSO4, filtered and concentrated under vacuum. Purification with flash chromatography (20 % Et2O/hexanes) afforded 12.82g (73.2 mmol, 44 % yield) of 4-tert-butoxybenzonitrile. 1H NMR (400 MHz, CDCl3) δ 7.54 (dq, J = 9.1, 2.3 Hz, 2H, Ar), 7.02 (m, 2H, Ar), 1.40 (s, 9H, tBu-CH3). 12.82 g of 4-tert-butoxybenzonitrile (73.2 mmol) was dissolved in 110 mL of Et2O and 72.8 mL of a solution of 1M LiAlH4 in THF added dropwise. After refluxing for 3 h, the mixture was brought back to room temperature and quenched slowly with 10 mL of water, 10 mL of aqueous 1M NaOH and finally 50 mL of water. The resulting slurry was filtered, the residue washed with Et2O and the filtrate concentrated under vacuum. Afterward, 50 mL of water was added to the crude and the pH adjusted to 2.7 using aqueous 1M KHSO4. The aqueous phase was washed with Et2O, the pH readjusted to 9-10 with aqueous 1M NaOH and extracted twice with DCM. Combined organic layers were washed with brine, dried with MgSO4, filtered and evaporated to dryness under vacuum to afford 12.53 g (69.9 mmol, 95 % yield) of 4-tert-butoxybenzylamine. 1H NMR (400 MHz, CDCl3) δ 7.13 (m, 2H, Ar), 6.89 (m, 2H, Ar), 3.75 (s, 2H, CH2), 1.28 (s, 9H, tBu-CH3). |
| 47.2% |
With lithium aluminium tetrahydride; In tetrahydrofuran; at 0℃; for 22h;Reflux; Inert atmosphere; |
175 mL of 1M (0360) (175 mmol; 2.6 eq.) lithium aluminum hydride in THF was added to a round bottom flask with a stir bar and was cooled to 0C. A solution of 11.83 g (67.5 mmol) of 4-(tert- butoxy)benzonitrile (Alfa Aesar, Ward Hill, MA) in 50 mL of anhydrous THF was added to the stirring solution dropwise over the course of 30 minutes. The reaction was then fitted with a reflux condenser and was heated to reflux for six hours, followed by stirring overnight under argon, allowing the reaction to cool to room temperature. After 22 hours of total reaction time, the reaction mixture was cooled to 0C and was quenched with 7 mL of water, followed by 6 mL of 15% NaOH (aq) and an additional 17 mL of water. The resulting emulsion was filtered over celite, with the filter cake being washed with methanol (2 x 50 mL) and DCM (2 x 50 mL). The filtrate was evaporated, and the resulting dark yellow oil was dissolved into 75 mL of water. The solution was transferred to a separatory funnel and was extracted with ethyl acetate (4 x 150 mL). The combined extractions were washed with water (100 mL) and brine (100 mL), dried over sodium sulfate, and evaporated in vacuo to give a dark yellow oil. The oil was separated using basic alumina chromatography and a solvent system of ethyl acetate/hexanes (20:80 to 50:50). The fractions containing the product (as evidenced by TLC) were combined and evaporated to give 5.71 g (47.2% yield) of 14 as a clear oil. lH NMR (300 MHz, CDC13) δ 7.19 (dd, J = 2.26, 6.46 Hz, 2H), 6.94 (dd, 7 = 2.32, 6.46 Hz, 2H), 4.38 (s, 2H), 1.31 (s, 9H). 13C NMR (75 MHz, CDCI3) δ 153.80, 135.32, 128.26, 124.27, 78.17, 54.93, 28.75. LRMS (EI) calculated mass (CnHisNO) (0361) [M+H]1+: 180.1388, mass found m/z: 180.1404 [M+H]1+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping