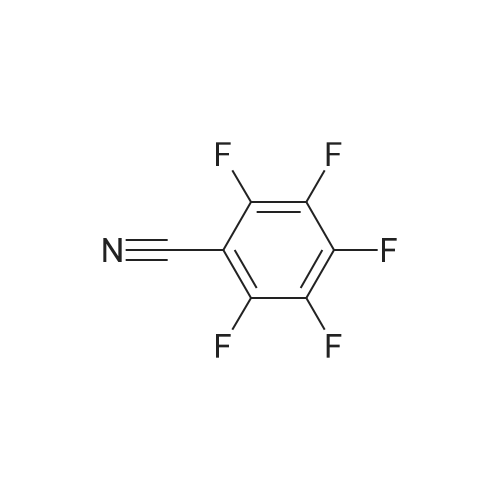
Metal Phthalocyanines Encapsulated in Faujasite Zeolites for Gas-Phase CO Oxidation
Iaia, Ethan P
;
Rana, Ganesh R
;
Soyemi, Ademola
, et al.
ACS Appl. Nano Mater.,2024,7(16):18824-18840.
DOI:
10.1021/acsanm.4c02406
More
Abstract: Existing metal-containing porous catalysts have inherent heterogeneity in metal species, rendering it difficult to compare reactivity across varied catalyst formulations without first developing active site quantification protocols. The supercages of faujasite zeolites (FAU) are large enough to confine metal phthalocyanines (MPCs), together serving as a well-defined active center for experimental and computational catalyst characterization. Deviations in zeolite synthesis conditions from prior literature were required to obtain phase-pure FAU. Metal perchloro-, perfluoro-, and perhydrogenated phthalocyanines (MPCCl16, MPCF16, and MPC; M = Cr, Mn, Fe, Co, Ni, Cu, and Zn) were encapsulated into FAU zeolites via hydrothermal synthesis (MPC@FAU) and deposited onto the external surfaces by postsynthetic deposition (MPC/FAU). These MPC@FAU catalysts were tested as catalysts for CO oxidation with dioxygen at 298 K and their reactivity compared to that of silica-supported PdAu nanoparticles and cobalt?nitrogen-doped carbon (Co?N?C). Initial CO2 site time yields were greater than the analogous metal-ion-exchanged zeolites (by ~50×). However, this initial activity decreased with time on stream for all MPC samples tested, and the cause of this deactivation is explored herein. Stable CO2 formation rates with time on stream observed over PdAu/SiO2 and Co?N?C suggest that deactivation observed over MPC@FAU samples is distinct and not an artifact of the experimental apparatus. Density functional theory calculations suggest an O2-activation mechanism, aided by the coadsorption of CO on the pyrrole N of the MPC and an axial ligand that can provide additional electron density to reduce the barrier for O2 bond breaking; this reaction mechanism is distinct from that over structurally similar metalnitrogen-doped carbons. Nevertheless, the reactivity of MPC@FAU catalysts for gas-phase CO oxidation with dioxygen at ambient temperature indicates that they may share similar functionality to metal?nitrogen-doped carbons and have the potential to serve as model catalysts for gas-phase chemistries.
Keywords:
molecular complexes ;
zeolites ;
oxidation ;
deactivation ;
carbon monoxide ;
mechanism
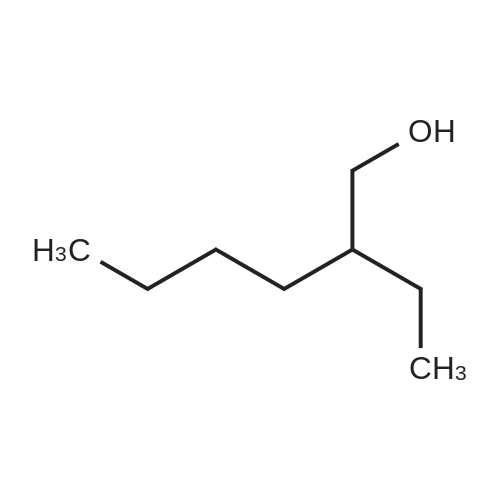
Purchased from AmBeed:
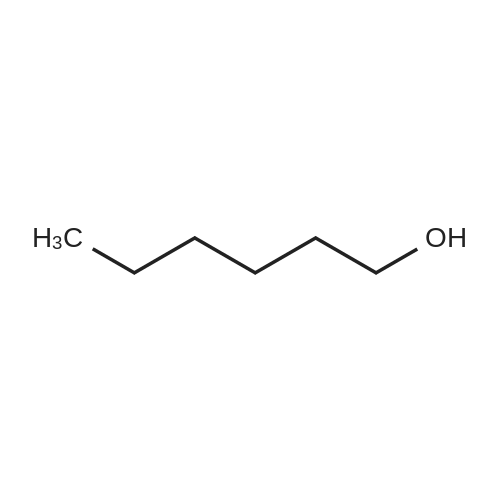
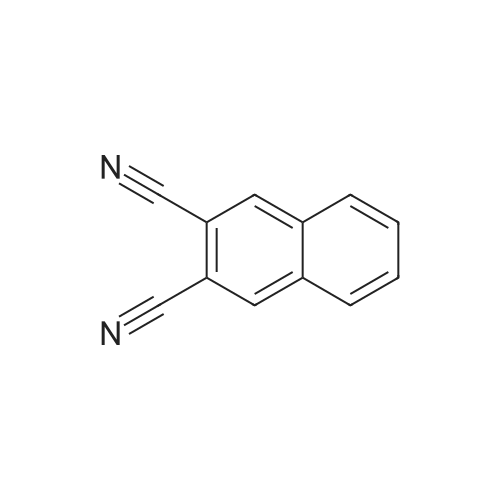
1835-65-0

(F5PhO)2-F16-SiPc as an air-stable, high-performance n-type semiconductor with poor cannabinoid sensing capabilities
Halynne R. Lamontagne
;
Mélanie Cyr
;
Mário C. Vebber
, et al.
RSC Appl. Interfaces,2024,1,1222-1232.
DOI:
10.1039/D4LF00147H
More
Abstract: Organic thin-film transistors (OTFTs) are an emerging platform for rapid, point-of-source detection and speciation of Δ9 -tetrahydrocannabinol (THC) and cannabidiol (CBD). (F5PhO)2-F16-SiPc semiconductor was implemented into high performance, air-stable n-type bottom gate, bottom contact OTFTs, however, the resulting device performance changes in response to THC and CBD were negligible. We explored the orientation of the corresponding thin films by synchrotron-based grazing incidence wide-angle X-ray scattering (GIWAXS) and angle-dependent near-edge X-ray absorption fine structure (NEXAFS), as well as polarized Raman microscopy. These techniques demonstrate for the first time that (F5PhO)2-F16-SiPc molecules are at a 45–48° orientation to the substrate; comparable to other reported R2-SiPcs. This orientation did not change upon exposure to THC and CBD, which has previously been reported for phthalocyanine-based OTFT cannabinoid sensors. The presence of two bulky axial groups, along with the absence of hydrogens in the molecule and the low reactivity of the silicon atom likely causes the lack of interaction with the cannabinoids. While (F5PhO)2-F16-SiPc may be a successfully air-stable n-type semiconductor for OTFTs, the structural changes performed to make it air stable over traditional nonfluorinated silicon MPcs, are likely responsible for its lack of response to cannabinoid exposure.
Purchased from AmBeed:
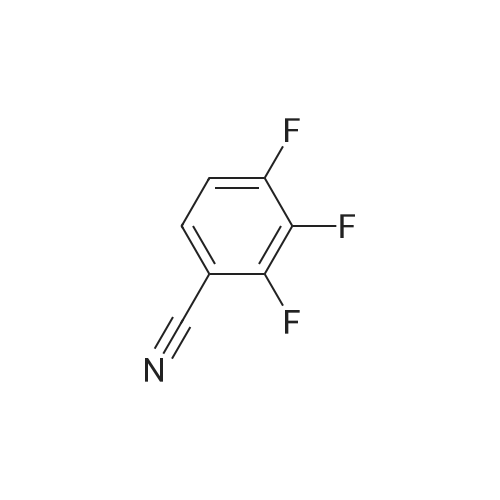
771-61-9 ;
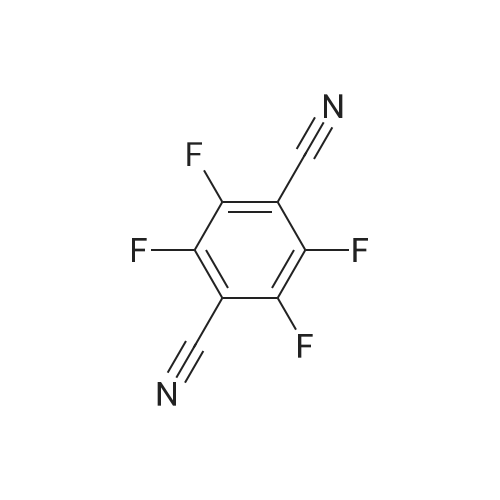
1835-65-0
2-F16-SiPc as an air-stable, high-performance n-type semiconductor with poor cannabinoid sensing capabilities.png)

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping