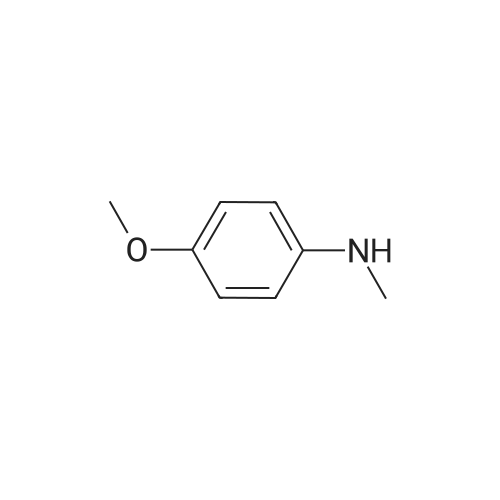
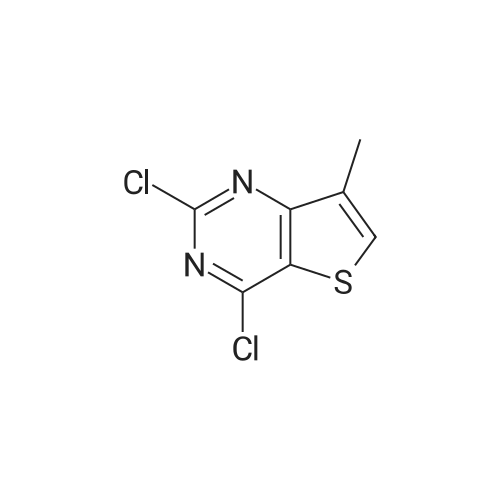
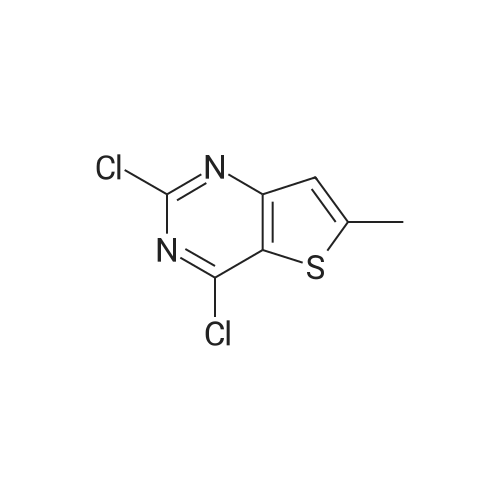
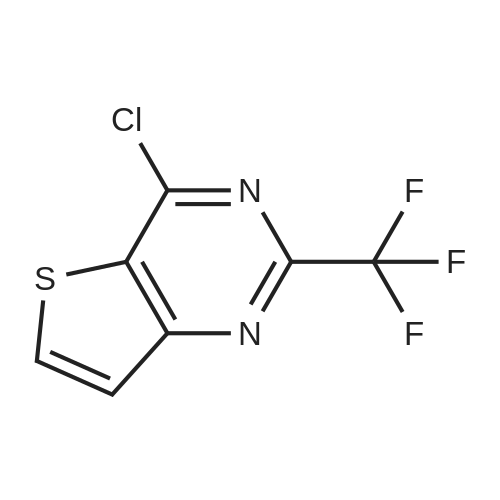
|
With acetic acid; In water; at 100℃; for 1h; |
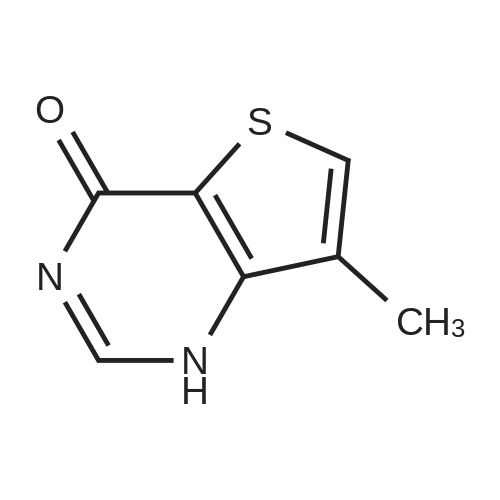
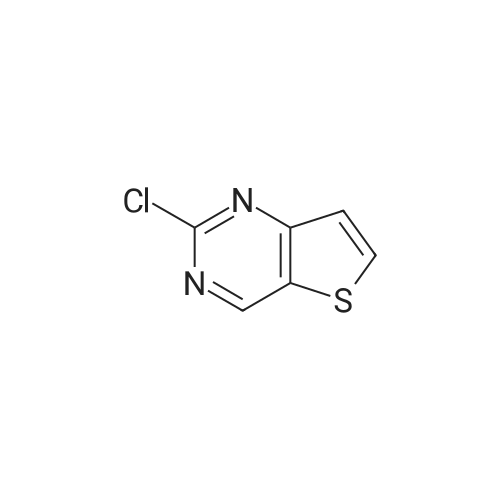
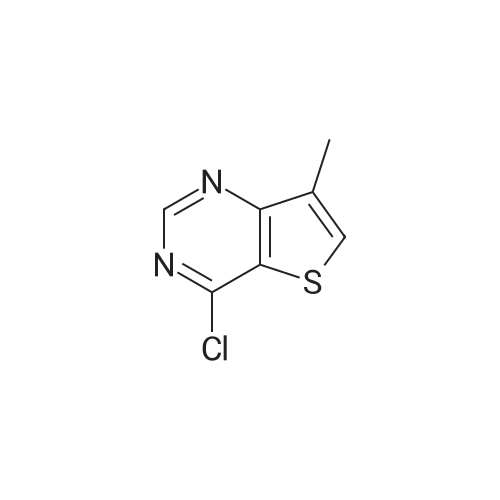
Example 81 : W-(6,6-dimethyl-5-[(3S,8aS)-3-methylhexahydropyrroIo[1,2-a]pyrazin-2(1H)- yl]carbonyl}-1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazol-3-yl)-7-methylthieno[3,2-d]pyrimidin-4-amine.Preparation of compound 81b: (3S,8aS)-3-methylhexahydropyrrolo[1,2-a]pyrazine-2(1H)-carbonyl chloride; To a stirring mixture of triphosgene (2.11g, 1eq) in CH2CI2 (10ml) at O0C was added DIPEA (1.8mi, 1.5eq) and (3S,8aS)-3-methyloctahydropyrrolo[1 ,2-a]pyrazine (81a, 1g, 7.13mmol). The resulting mixture was stirred at O0C for 30 min. The reaction mixture was evaporated in vacuo to give a residue, compound 81b, which was directly carried onto the next reaction without further purification. EPO <DP n="83"/>Preparation of compound 81c: ethyl 3-amino-6,6-dimethyl-5-[(3S,8aS)-3- methylhexahydropyrrolo?^-alpyrazin-atifO-yllcarbony^-δ.β-dihydropyrroloIS^-clpyrazoIe^t^- carboxylate; To a stirring mixture of compound I(H) 5-fe/f-butyl 2-ethyl 3-amino-6,6-dimethylpyrrolo[3,4-c]pyrazole- 2,5(4H,6H)-dicarboxylate (5.65g, 17.4mmol) in CH2CI2 (20ml) was added 4.0M HCI in dioxane (30ml). The reaction mixture was concentrated in vacuo to give crude HCI salt of compound 54a. A portion of residue (54a, 1g, 4.46mmol) was added to a stirring mixture of (3S,8aS)-3-methylhexahydropyrrolo[1,2- a]pyrazine-2(1W)-carbonyl chloride (81b, 1.4 g, 2eq) in CH2CI2 (20ml), DIPEA (1.2ml, 2eq). The resulting mixture was stirred at room temperature for 15h. The reaction mixture was diluted with CH2CI2, and washed with saturated NaHCO3, dried over sodium sulfate, concentrated in vacuo, purified by flash chromatography. Elution with 5-15% MeOH/DCM provided compound 81c. 1H NMR (CD3)2SO δ: 1.2 (m, 2 H), 1.31 (t, 3 H), 1.52 (m, 6H), 1.64 (m, 4H), 1.93 (m, 1H), 2.18 (m, 1H), 2.77 (m, 2H), 2.93 (m, 1H), 3.77 (m, 1H), 4.18 (m, 2H), 4.33 (m, 2H) Preparation of compound 81 d: 6,6-dimethyl-5-[(3S,8aS)-3-methylhexahydropyrrolo[1,2-a]pyrazin- 2(1 rt)-yl]carbonyl}-1 ,4,5,6-tetrahydropyrrolo[3,4-c]pyrazol-3-amineTo a stirring solution of ethyl 3-amino-6,6- dimethyl- 5-[(3S,8aS)-3-methyl hexahydropyrrolo [1,2- a]pyrazin-2(1H)-yl]carbonyl}-5,6-dihydropyrrolo[3,4-c] pyrazole-2(4W)- carboxylate (81c, 613mg, 1.δOmmol) in MeOH (3mL) was added 20% aq. NaOH (2ml). The resulting mixture was stirred at room temperature for 30 min. The reaction mixture was concentrated and the residue was partitioned between ethyl acetate and saturated NaHCO3, dried, and concentrated to give compound 81 d.To a stirring solution of compound 81d (0.15Og, 0.47mmol) in 50% acetic acid / water (4ml) was added 4- chloro-7-methylthieno [3,2-cfl pyrimidine (175 mg, 2eq). The resulting mixture was heated to a temperature of 100C for 1 hr. The reaction mixture was purified by prep-HPLC to provide compound 81 as a white solid 1H NMR (CD3)2SO δ: 1.23 (m, 2 H), 1.62 (d, 6 H), 1.69 (m, 3 H), 1.83 (m, 1 H), 1.95 (m, 1 H), 2.16 (m, 1 H), 2.34 (s, 3H), 2.74 (m, 2H), 2.90 (m, 1H), 3.80 (m, 1 H), 4.52 (s, 2H), 7.83 (s, 1H), 8.56 (s, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping