|
In hexane; toluene; for 48.5h;Heating / reflux; |
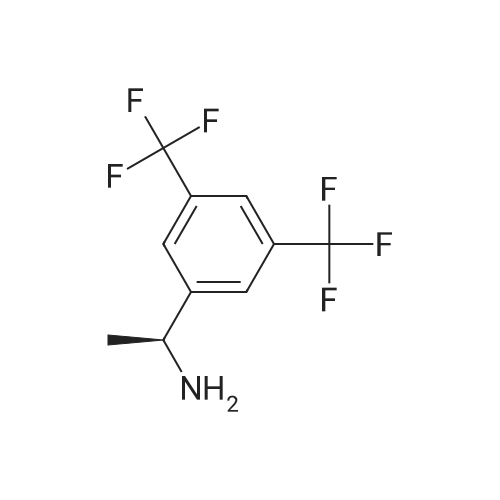
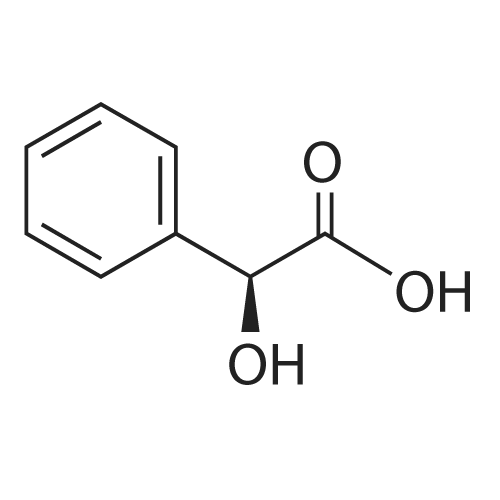
0.64 g (2.49 mmol, 1 eq) of optically active 1-(3,5-bis-trifluoromethylphenyl)ethylamine (5a, enantiomer ratio/S form:R form=3.8:1) and 0.17 g (1.12 mmol, 0.45 eq) of (S)-mandelic acid were added to 3 ml of toluene, followed by stirring for 30 minutes under reflux, the addition of 1.5 ml of n-hexane and allowing to cool to room temperature and stand for 2 days in a refrigerator. The precipitated crystals were filtered, washed with a small amount of n-hexane and vacuum dried to obtain 0.53 g of crystals having the structure represented by the formula below and 0.28 g of mother liquor. They were converted to the free bases with 0.5 N aqueous NaOH and analyzed by chiral GC. With this, respective ee were found to be 96.1% ee (major form is the S form) and 4.7% ee (major form is the S form). [C00041] [00184] 1H-NMR (TMS, DMSO): 1.39 (d, 6.8 Hz, 3H), 4.41 (q, 6.5 Hz, 1H), 4.71 (d, 2.0 Hz, 1H), 7.19 (t, 7.3 Hz, 1H), 7.26 (t, 7.3 Hz, 2H), 7.36 (d, 7.3 Hz, 2H), 8.01 (s, 1H), 8.15 (s, 2H).Example 33Recrystallization Purification by (s)-Mandelic Acid Salt of Optically Active 1-(3.5-Bis-trifluoromethylphenyl)ethylamine (5a) [00185] 0.80 g (3.10 mmol, 1 eq) of optically active 1-(3,5-bis-trifluoromethylphenyl)ethylamine (5a, enantiomer ratio/S form:R form=8.8:1) and 0.47 g (3.09 mmol, 1 eq) of (S)-mandelic acid were added to 4.5 ml of toluene, followed by stirring for 30 minutes under reflux, the addition of 1.8 ml of n-hexane, allowing to cool to room temperature, adding seed crystals and allowing to stand for 3 hours. The precipitated crystals were filtered, washed with a small amount of a-hexane and vacuum dried to obtain 0.89 g of crystals having the structure represented by the formula below and 0.35 g of mother liquor. They were converted to the free bases with 0.5 N aqueous NaOH and analyzed by chiral GC. With this, respective ee were found to be 90.7% ee (major form is the S form) and 58.1% ee (major form is the S form). [C00042] [00186] The 1H-NMR spectrum was the same as that of Example 32. |
|
In toluene; at 20 - 80℃; for 1.5h;Heating; |
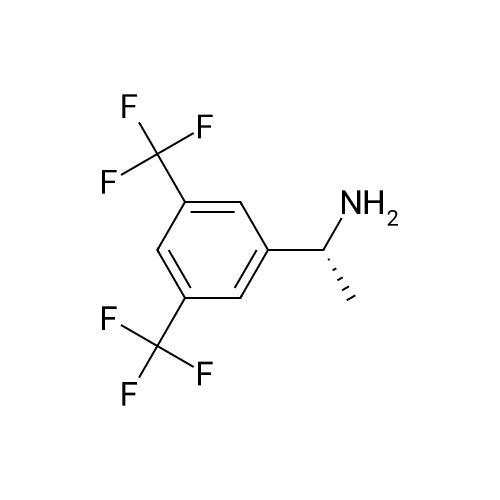
0.89 g of (S)-mandelic acid salt of optically active 1-(3,5-bis-trifluoromethylphenyl)ethylamine (5a) (5a (S)-mandelate, enantiomer ratio/S form:R form=95.5:4.5) were added to 10 ml of toluene, followed by stirring for 30 minutes at 80 C. and allowing to cool to room temperature and stand for 1 hour. The precipitated crystals were filtered, washed with a small amount of toluene and vacuum dried to obtain 0.71 g of crystals having the structure represented by the formula below and 0.18 g of mother liquor. They were converted to the free bases with 0.5 N aqueous NaOH and analyzed by chiral GC. With this, respective ee were found to be 99.7% ee (major form is the S form) and 82.7% ee (major form is the S form). [C00043] [00188] The 1H-NMR spectrum was the same as that of Example 32. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping