|
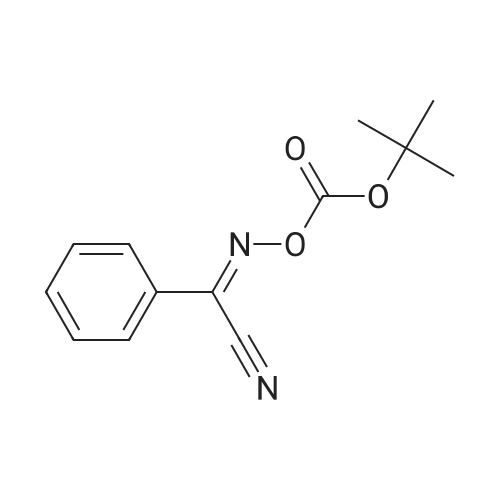
With potassium carbonate; In diethyl ether; water; at 20℃; for 16h; |
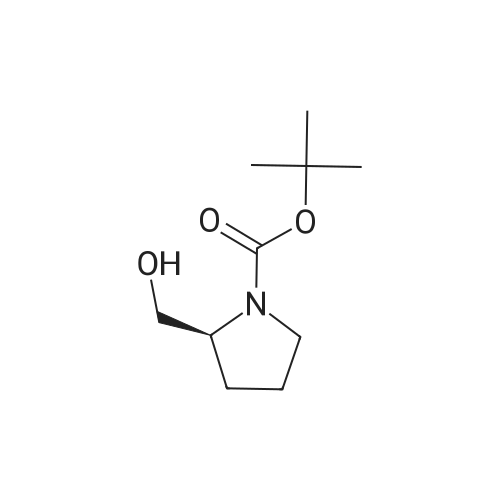
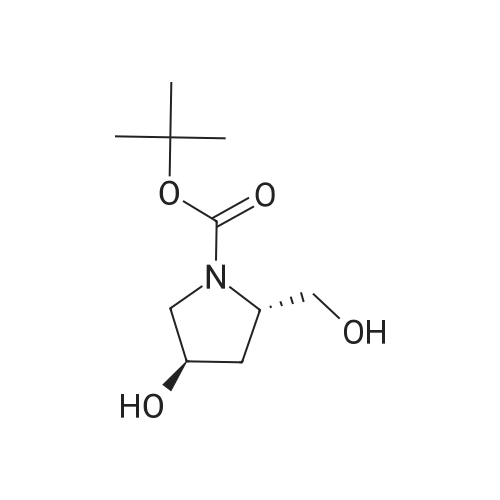
To a solution of DL-proline (10.0 g, 86.9 mmol) in THF (20 mL) were added boron trifluoride etherate complex (12.9 g, 91.2 mmol) and borane-tetrahydrofuran (1.0 mol/L THF solution, 100 mL) at 0°C, and the mixture was stirred at room temperature for 16 hr. After completion of the reaction, the mixture was further heated under reflux for 1 hr and cooled to room temperature. THF-water (1:1, 2.5 mL) and 6N sodium hydroxide were successively added to the reaction solution, and the mixture was heated under reflux for 2 hr. The reaction solution was cooled to room temperature, and concentrated under reduced pressure. The residue was washed with diethyl ether. The remaining residue, di-tert-butyl dicarbonate (19.9 g, 91.2 mmol) and potassium carbonate (36.0 g, 260 mmol) were dissolved in diethyl ether-water (100 mL-150 mL), and the mixture was stirred at room temperature for 16 hr. The diethyl ether layer was separated and washed with saturated brine, dried over anhydrous magnesium sulfate, and filtered. The filtrate was concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography (hexane-ethyl acetate 95:5 - 60:40 - 50:50) to give the title compound (13.6 g, 76percent) as a colorless oil. 1H NMR (300 MHz, CDCl3) delta 4.74 (d like, 1H), 3.96 (br s, 1H), 3.74-3.21 (m, 4H), 2.13-1.67 (m, 4H), 1.49 (s, 9H). |
|
|
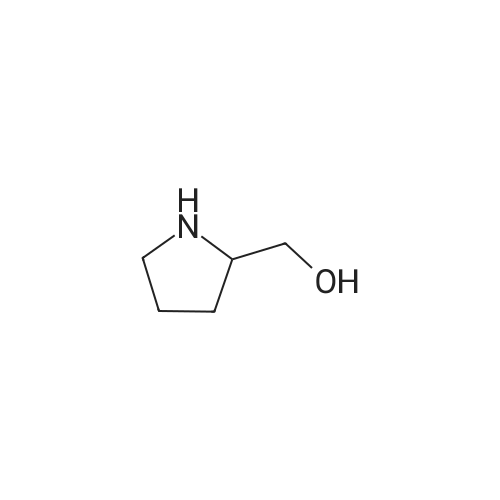
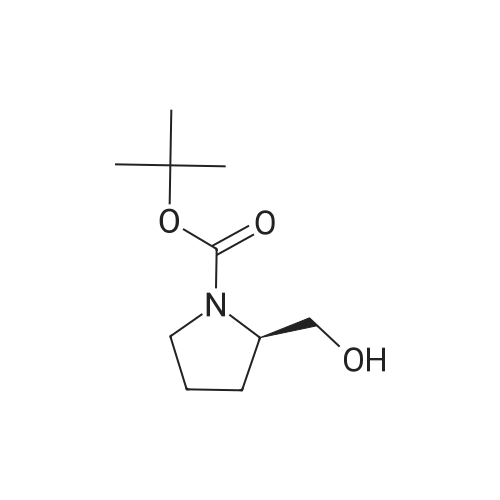
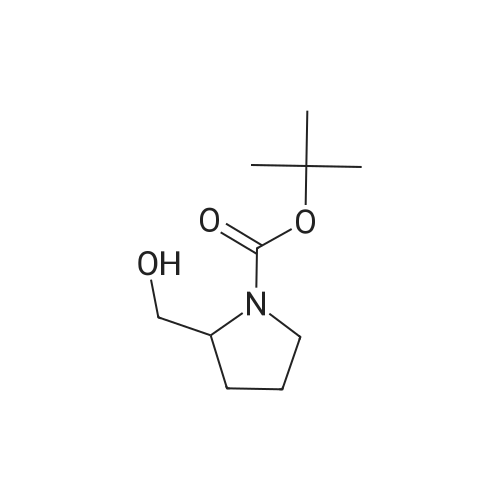
Maleic acid (2.86g, 24.72 mmol) was added to pyrrolidin-2-ylmethanol(2.5g, 24.72 mmol) (A.170) in ethyl acetate (10 mL). The resultant mixture was stirred at room temperature for 2 hrs. After concentration, NaHCO3(10.38g, 123.6 mmol) in water (16 mL) was added to the residue. (BOC)2O (6.47 g, 29.66 mmol) was then added. The mixture was stirred at room temperature for 16 hrs. After removing solid by filtration, the aqueous solution was extracted with ethyl acetate. The organics were dried and concentrated, gave crude N-BOC protected pyrrolidin-2-ylmethanol. To the crude N-BOC protected pyrrolidin-2-ylmethanol(2.5g, 12.43mmol) and triethylamine (4.16ml, 29.82 mmol) in ethyl acetate at O0C, MsCl(1.16 mL, 14.91 mmol) was added and the mixture was stirred at 0°C for 2 hrs. The reaction was quenched with water and extracted with ethyl acetate. The organics were washed with 2N HCl, water, Sat. aq. NaHCO3, and brine, dried and concentrated. A portion of the residue(257.6 mg, 0.924mmol) was added to a mixture Of Cs2CO3 ( 301.1 mg, 0.924 mmol) and 4-(5-bromo-lH-indol-3-yl)-5-chloropyrimidin-2-amine A.170 (100 mg, 0.308 mmol) in DMF (1 mL) and DMSO(I mL). The mixture was heated at 110 0C for 16 hrs. After cooling to room temperature, the mixture was diluted with ether, washed with water and brine, dried and concentrated. A portion of the residue (68.5mg, 0.135 mmol) was dissolved in dichloromethane (8 mL) and TFA (2mL) then was added. The mixture was stirred at room temperature for 1 hr, concentrated and diluted with methanol-dichloromethane (1 :9). The solution was washed with Sat. aq. NaHCO3, and brine, dried and concentrated to afford 4-(5- bromo-l-(pyrrolidin-2-ylmethyl)-lH-indol-3-yl)-5-chloropyrimidin-2-amine (A.178) (32.0 mg, 58percent): 1H NMR (methanol- d4) b 8.78(s, 1 H), 8.43(s, 1 H), 8.15(s, 1 H), 7.42 (d, J= 8.0 Hz, 1 <n="98"/>H), 7.38(d, J= 8.0 Hz, 1 H), 4.29-4.15(m, 2 H), 3.55-3.45(m, 1 H), 3.00-2.95(m, 1 H), 2.90- 2.85(m, 1 H)), 1.92-1.80(m, 2 H), 1.80-1.72(m, 1 H), 1.55-1.46(m, 1 H); ms 406.0 (M+H+). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping